Figure 2
Transglutaminase inhibition: possible therapeutic mechanisms to protect cells from death in neurological disorders
Vittorio Gentile*, Elenamaria Fioretti, Nicola Gaetano Gatta and Rosaria Romano
Published: 25 July, 2017 | Volume 1 - Issue 1 | Pages: 026-038
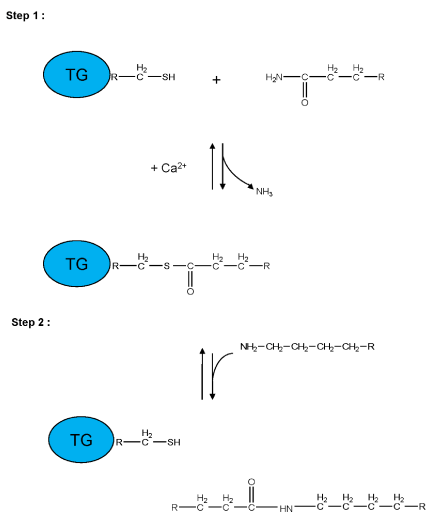
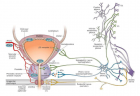
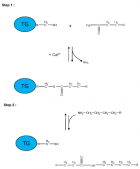
Figure 2:
Schematic representation of a two-step transglutaminase reaction. Step 1: In the presence of Ca2+, the active-site cysteine residue reacts with the γ-carboxamide group of an incoming glutaminyl residue of a protein/peptide substrate to yield a thioacyl-enzyme intermediate and ammonia. Step 2: The thioacyl-enzyme intermediate reacts with a nucleophilic primary amine substrate, resulting in the covalent attachment of the amine-containing donor to the substrate glutaminyl acceptor and regeneration of the cysteinyl residue at the active site. If the primary amine is donated by the ε-amino group of a lysyl residue in a protein/polypeptide, a Nε-(γ-L-glutamyl)-L-lysine (GGEL) isopeptide bond is formed.
Read Full Article HTML DOI: 10.29328/journal.hjbm.1001004 Cite this Article Read Full Article PDF
More Images
Similar Articles
-
Transglutaminase inhibition: possible therapeutic mechanisms to protect cells from death in neurological disordersVittorio Gentile*,Elenamaria Fioretti,Nicola Gaetano Gatta,Rosaria Romano. Transglutaminase inhibition: possible therapeutic mechanisms to protect cells from death in neurological disorders . . 2017 doi: 10.29328/journal.hjbm.1001004; 1: 026-038
Recently Viewed
-
Forensic Psychology and Criminal ProfilingEze SM*,Alabi KJ,Yusuf AO,Hamzat FO,A Abdulrauf,Atoyebi AT,Lawal IA,OA Ibrahim,AY Imam-Fulani,Dare BJ. Forensic Psychology and Criminal Profiling. J Forensic Sci Res. 2025: doi: 10.29328/journal.jfsr.1001085; 9: 092-096
-
Forensic Insights into Multiple Stab Wounds: Autopsy Findings from a Case of Sixty Stab WoundsAsif Hussain*. Forensic Insights into Multiple Stab Wounds: Autopsy Findings from a Case of Sixty Stab Wounds. J Forensic Sci Res. 2025: doi: 10.29328/journal.jfsr.1001075; 9: 021-024
-
The Use and Prevalence of Cannabis among Students of Nnamdi Azikiwe University, Awka, Anambra StateChijioke M Ofomata*,Enyinna P Nnabuihe. The Use and Prevalence of Cannabis among Students of Nnamdi Azikiwe University, Awka, Anambra State. J Forensic Sci Res. 2025: doi: 10.29328/journal.jfsr.1001088; 9: 104-108
-
Fact-finding Investigation for the Activation of the Legal-forensic Nursing Consultancy Desk at the Order of Nursing Professions of the Province of AvellinoA Masucci*,AA Di Gisi,A De Sapio. Fact-finding Investigation for the Activation of the Legal-forensic Nursing Consultancy Desk at the Order of Nursing Professions of the Province of Avellino. J Forensic Sci Res. 2025: doi: 10.29328/journal.jfsr.1001086; 9: 097-099
-
Critical and Comparative Analysis of the 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for Hypertension and Other International Guides and ProtocolsLuis Alcocer*,Martin Rosas-Peralta*,Héctor Galván-Oseguera,Humberto Álvarez-López,Ernesto Cardona-Muñoz,Adolfo Chávez-Mendoza,Silvia Palomo-Piñón,Enrique Díaz Díaz,José Manuel Enciso-Muñoz. Critical and Comparative Analysis of the 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for Hypertension and Other International Guides and Protocols. Ann Clin Hypertens. 2025: doi: 10.29328/journal.ach.1001040; 9: 018-00
Most Viewed
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215
-
Prospective Coronavirus Liver Effects: Available KnowledgeAvishek Mandal*. Prospective Coronavirus Liver Effects: Available Knowledge. Ann Clin Gastroenterol Hepatol. 2023 doi: 10.29328/journal.acgh.1001039; 7: 001-010
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."