About Institute for Research in Biotechnology
Institute for Research in Biotechnology
Articles by Institute for Research in Biotechnology
Anti-Inflammatory probiotic biomarkers in Fermented foods
Published on: 24th January, 2019
OCLC Number/Unique Identifier: 7985918960
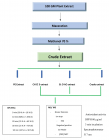
We present below a mechanistic molecular approach for development of Anti-Inflammatory biomarkers of Probiotic Bacteria in Fermented Foods. Probiotics are live microorganisms that promote human health by counteracting the noxious toxic gut microflora in human intestine, by modulating of the tight junctions, and by increasing mucin production, enforcing intestinal epithelial cell barrier function, modifying microbial community within the gut intestinal disorders, and improving immune responses associated with chronic inflammation in experimental animal models, collectively enhancing human health. Cytokine secretion by intestinal epithelial cells and macrophages are regulated by probiotics through key signaling pathways such as nuclear factor-κB and mitogen-activated kinases, resulting in alleviation of several disorders such as allergies, diabetes, obesity, heart diseases and cancer. MicroRNAs are small non-coding RNA molecules involved in transcriptional and post-translational regulation of gene expression by inhibiting gene translation. Using in vitro and in vivo approaches in cell lines and mice models to study effects of probiotic conditional media and heat-killed bacterial strains with anti-inflammatory effect to elucidate the mechanisms by which probiotics affect signaling pathways, and by using global cytokine and microRNA gene expression analyses arrroaches to develop biomarkers for studying different pro- and anti-inflammatory activities, and using statistical approaches to analyse the data, we show that cytokines and miRNAs have an essential role in regulation of cancerous and inflammatory bathways. This mechanistic approach will result in developing specific disease biomarkers for the early diagnosis of certain pathogenic states, as well as evaluating the effect of different dietary componenents on developed biomarkers in health states that will promote and enhance human health. Comparing the concordance of the in vitro to the in vivo research findings will confirm the correspondence of both approaches to each other. Moreover, this study will have a major public health relevance in elucidating the role of miRNAs and their targets in inflammation, paving the way to diagnosing and treating of pathogenic human disease stages.
Use of MicroRNAs to Screen for Colon Cancer
Published on: 31st August, 2017
OCLC Number/Unique Identifier: 7317598451
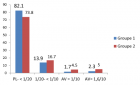
Colon cancer (CC) screening is important for diagnosing early stage for malignancy and therefore potentially reduces mortality from this disease because the cancer could be cured at the early disease stage. Early detection is needed if accurate and cost effective diagnostic methods are available. Mortality from colon cancer is theoretically preventable through screening. The Current screening method, the immunological fecal occult blood test, FOBTi, lacks sensitivity and requires dietary restriction, which impedes compliance. Moreover colonoscopy is invasive and costly, which decreases compliance, and in certain cases could lead to mortality. Compared to the FOBT test, a noninvasive sensitive screen that does not require dietary restriction would be more convenient. Colonoscopy screening is recommended for colorectal cancer (CRC). Although it is a reliable screening method, colonoscopy is an invasive test, often accompanied by abdominal pain, has potential complications and has high cost, which have hampered its application worldwide.
A screening approach that uses the relatively stable and nondegradable microRNA molecules when extracted from either the noninvasive human stool, or the semi-invasive blood samples by available commercial kits and manipulated thereafter, would be more preferable than a transcriptomic messenger (m)RNA-, a mutation DNA-, an epigenetic-or a proteomic-based test. That approach utilizes reverse transcriptase (RT), followed by a modified quantitative real-time polymerase chain reaction (qPCR). To compensate for exosomal miRNAs that would not be measured, a parallel test could be performed on stool or plasma’s total RNAs, and corrections for exosomal loss are made to obtain accurate results. Ultimately, a chip would be developed to facilitate diagnosis, as has been carried out for the quantification of genetically modified organisms (GMOs) in foods. The gold standard to which the miRNA test is compared to is colonoscopy. If laboratory performance criteria are met, a miRNA test in human stool or blood samples based on high throughput automated technologies and quantitative expression measurements currently employed in the diagnostic clinical laboratory, would eventually be advanced to the clinical setting, making a noticeable impact on the prevention of colon cancer.

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."