More Information
Submitted: June 18, 2024 | Approved: June 25, 2024 | Published: June 28, 2024
How to cite this article: Arif A, Garg P, Srivastava P. Microbiome-Gut-Brain Axis: AI Insights. Insights Biol Med. 2024; 8: 001-010.
DOI: 10.29328/journal.ibm.1001027
Copyright License: © 2024 Arif A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Microbiome-Gut-Brain Axis; Artificial intelligence (AI); Dysbiosis; Predictive modeling; Biomarker discovery
Microbiome-Gut-Brain Axis: AI Insights
Amaan Arif, Prekshi Garg and Prachi Srivastava*
Amity Institute of Biotechnology, Amity University Uttar Pradesh, Lucknow Campus, 226028, India
*Address for Correspondence: Prachi Srivastava, Amity Institute of Biotechnology, Amity University Uttar Pradesh, Lucknow Campus, 226028, India, Email: [email protected]
Microbiome-gut-brain axis represents a complex, bidirectional communication network connecting the gastrointestinal tract and its microbial populations with the central nervous system (CNS). This complex system is important for maintaining physiological homeostasis and has significant implications for mental health. The human gut has trillions of microorganisms, collectively termed gut microbiota, which play important roles in digestion, immune function, and production of various metabolites. Some current research shows that these microorganisms strongly influence the brain function and behaviour of individuals, forming the basis of the microbiome-gut-brain axis. The communication between gut microbiota and the brain occurs via multiple pathways: neural pathway (e.g., vagus nerve), endocrine pathway (e.g., hormone production), immune pathway (e.g., inflammation modulation), and metabolic pathway (e.g., production of short-chain fatty acids). Dysbiosis, or imbalance of gut microbiota, has been linked to mental health disorders such as anxiety, depression, multiple sclerosis, autism spectrum disorders, etc, offering new perspectives on their etiology and potential therapeutic interventions. Artificial Intelligence (AI) has emerged as a powerful tool in interpreting the complexities of the microbiome-gut-brain axis. AI techniques, such as machine learning and deep learning, enable the integration and analysis of large, multifaceted datasets, uncovering patterns and correlations that can be avoided by traditional methods. These techniques enable predictive modeling, biomarker discovery, and understanding of underlying biological mechanisms, enhancing research efficiency and covering ways for personalized therapeutic approaches. The application of AI in microbiome research has provided valuable insights into mental health conditions. AI models have identified specific gut bacteria linked to disease, offered predictive models, and discovered distinct microbiome signatures associated with specific diseases. Integrating AI with microbiome research holds promise for revolutionizing mental health care, offering new diagnostic tools and targeted therapies. Challenges remain, but the potential benefits of AI-driven insights into microbiome-gut-brain interactions are immense and offer hope for innovative treatments and preventative measures to improve mental health outcomes.
Overview of microbiome-gut-brain axis and its significance in mental health
Microbiomes-gut-brain axis is a bidirectional and dynamic communication network that links the gastrointestinal tract and its microbial community/ populations with the central nervous system (CNS). This complex system is important for maintaining homeostasis and has been increasingly recognized for its significant influence on mental health [1-3].
The microbiomes-gut-brain axis: The human gut is a home/host of trillions of microorganisms, collectively known as the gut microbiota of individuals. These microorganisms play important for various physiological processes including digestion, immune function, and the production of various metabolites. Recent research has highlighted the significant impact of these gut microbiota on brain function and behaviour, thereby forming the microbiome-gut-brain axis. This axis facilitates complex communication between the gut and brain through multiple interconnected pathways [3].
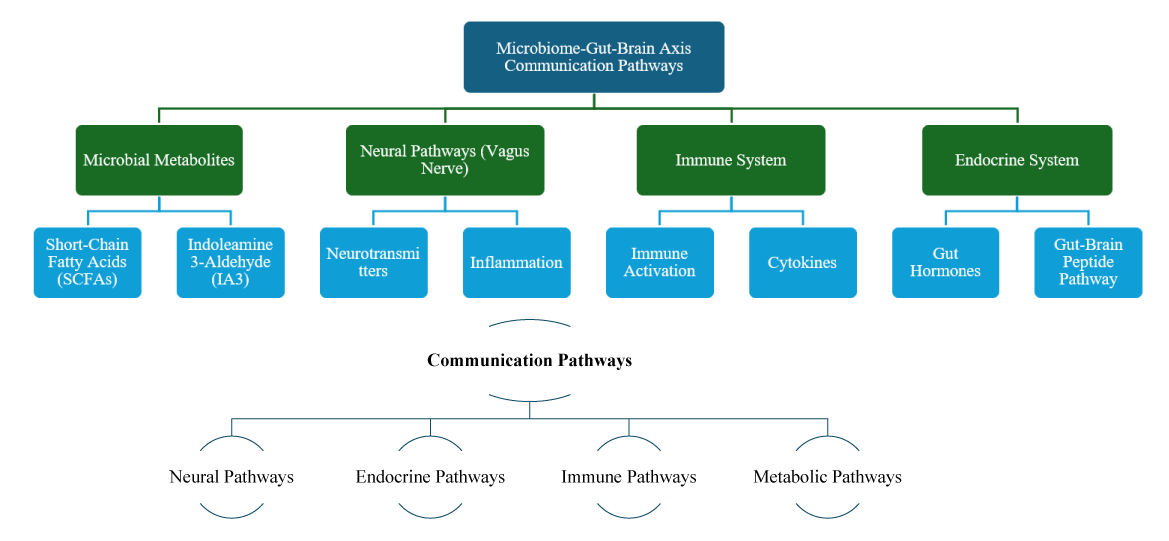
Communication pathways: The communication between gut microbiota and brain occurs through multiple pathways, each contributing to the overall function of the microbiome-gut-brain axis (Figure 1):
Figure 1: Communication Pathways in the Microbiome-Gut-Brain Axis.
Neural pathways: The Vagus nerve, which connects the gut to the brain, is a primary route for transmitting signals transmission. Gut bacteria can influence the vagus nerve, thereby affecting brain activity and behavior. This neural pathway enables rapid and direct communication between the gut and the CNS [4,5].
Endocrine pathways: Gut microbiota can modulate the production of hormones such as cortisol, which is involved in stress response and mood regulation. By influencing hormone levels, gut microbiota can impact emotional and psychological states [6].
Immune pathways: Gut microbiota interacts with the immune system, influencing the inflammation process and immune responses that can affect brain health. Chronic inflammation and immune dysregulation are linked to various mental health disorders, emphasizing the importance of this pathway [7,8].
Metabolic pathways: Gut bacteria produce metabolites, such as short-chain fatty acid (SCFA), which can able to cross the blood-brain barrier and influence brain functions. These metabolites play a role in modulating neural activity and neuroinflammation, contributing to overall brain health [9,10].
Significance in mental health: The microbiome-gut-brain axis has deep associations with mental health. Dysbiosis, or imbalances of gut microbiota, has been associated with various mental health disorders, such as anxiety, depression, multiple sclerosis, and autism spectrum disorder. Understanding the microbiomes-gut-brain axis mechanism offers new perspectives on the etiology of these conditions and opens up potential avenues for therapeutic interventions [11].
The significance of the microbiomes-gut-brain axis in mental health is multifaceted. For example, alterations in gut microbiota composition can lead to changes in the production of neurotransmitters and other neuroactive compounds, thereby affecting mood and behavior. Moreover, modulation of gut microbiota through diet, probiotics, or prebiotics holds promise for developing novel treatments for mental health disorders [11].
Advances in Artificial Intelligence (AI) are enhancing our understanding of this complex multifaceted system. AI algorithms can analyze microbiome datasets to identify patterns/signatures, and predict outcomes, thus helping in deeper insights into the microbiome-gut-brain axis. AI is a main field, that encompasses both ML and DL. ML uses algorithms to learn from data and make predictions while DL a subset of ML uses neural networks with multiple layers to analyze large and complex datasets. These technologies help us in the identification of complex patterns and relationships within microbiome data, enhancing our understanding of its impact on brain health. By using AI, researchers can uncover novel biomarkers and therapeutic targets, paving the way for innovative treatments and preventative measures for mental health disorders, potentially revolutionizing mental health care [12].
Importance of AI in understanding the microbiome-gut-brain axis
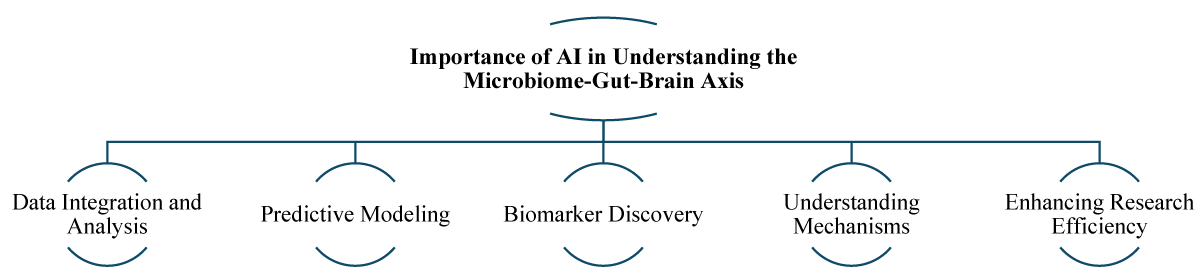
Microbiome-gut-brain axis is a dynamic and complex system, involving complex interactions between gut microbiota and the brain. Understanding these interactions is crucial for advancing our knowledge of mental health. AI technologies have transformed many fields, and their application in microbiome research is particularly significant [11]. Here’s how AI contributes to understanding the complex interactions between gut microbiota and the brain (Figure 2).
Figure 2: Importance of AI in Understanding Microbiome-Gut-Brain Axis.
Data integration and analysis: The microbiome-gut-brain axis involves vast and multifaceted data, including genetic, metabolic, and neuroimaging information. AI algorithms, such as machine learning and deep learning, can integrate and analyze these diverse datasets. They identify patterns and correlations that are beyond human analytical capabilities, revealing new insights into how gut microbiota influence brain function and behavior [13].
Predictive modeling: Understanding the link between changes in gut microbiota and mental health outcomes is a challenging task due to the high variability and dynamic nature of microbiome compositions. AI-based predictive modeling can simulate various scenarios to help researchers understand the potential outcomes of microbiota changes. These predictive models can forecast responses to interventions such as dietary modifications, probiotics, and other treatments, thus facilitating the development of personalized medicine approaches customized to individual patients [14]. For instance, predictive models might suggest how specific probiotic strains could alleviate depressive symptoms in certain individuals [15].
Biomarker discovery: Identifying specific biomarkers that establish a connection between gut microbiota and mental health conditions presents a significant challenge due to the complex and personalized nature of these interactions. AI techniques can efficiently sift through large datasets to discover biomarkers associated with specific mental health conditions. This is because different individuals may have unique interactions between their gut microbiota and mental health, making it challenging to identify universal biomarkers. These biomarkers can serve as diagnostic tools or targets for new therapies. For example, AI might identify a particular bacterial metabolite that is consistently low in individuals with anxiety, suggesting it as a potential therapeutic target [16,17].
Understanding mechanisms: It is well-established that gut microbiota has a significant impact on brain function, but the specific biological mechanisms through which gut microbiota affect brain function are not fully understood. Using advanced analytical techniques such as genetic sequencing, proteomic analysis, and metabolomic profiling, researchers can use AI to gain deeper analysis into how gut microbiota influences brain function. AI can help uncover complex details such as specific neuroactive compounds produced by gut bacteria, their role in modulating immune responses, and their interactions with the nervous system. This multifaceted approach holds great promise for advancing our understanding of the complex relationship between gut microbiota and brain function. For instance, AI might uncover how certain gut bacteria produce neurotransmitters like serotonin, which play a critical role in mood regulation [18,19].
Enhancing research efficiency: Research methods have traditionally been time-consuming and limited in terms of scope. However, with the introduction of AI, research processes have been significantly accelerated. AI plays an important role in automating data analysis and generating hypotheses for further exploration. This automation allows for a substantial increase in efficiency and enables more rapid advancements in understanding the microbiome-gut-brain axis. For example, AI-driven analysis can quickly identify promising research avenues that human researchers might overlook, thereby expediting the discovery process [20,21].
AI plays an important role in deciphering complex interactions between gut microbiota and the brain. By integrating and analyzing vast amounts of data, predicting outcomes, discovering biomarkers, and understanding underlying mechanisms, AI provides valuable insights that are essential for advancing mental health research. The application of AI in this field not only enhances our understanding but also opens up new avenues for innovative therapeutic strategies.
AI techniques in microbiome-gut-brain research
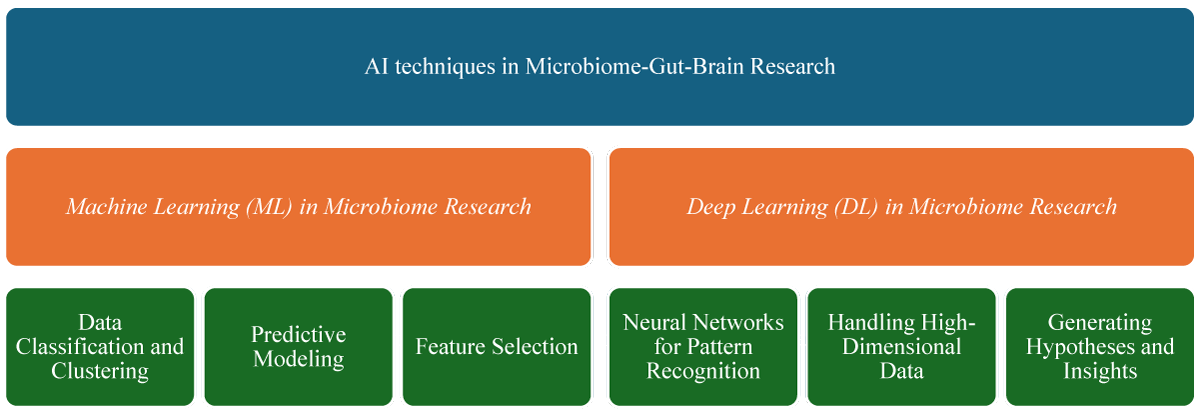
Advancements in Artificial Intelligence (AI), particularly in Machine Learning (ML) and Deep Learning (DL), have significantly enhanced our ability to analyze and interpret complex microbiome data. These techniques provide powerful tools for understanding the microbiome-gut-brain axis, offering deeper insights into its influence on mental health.
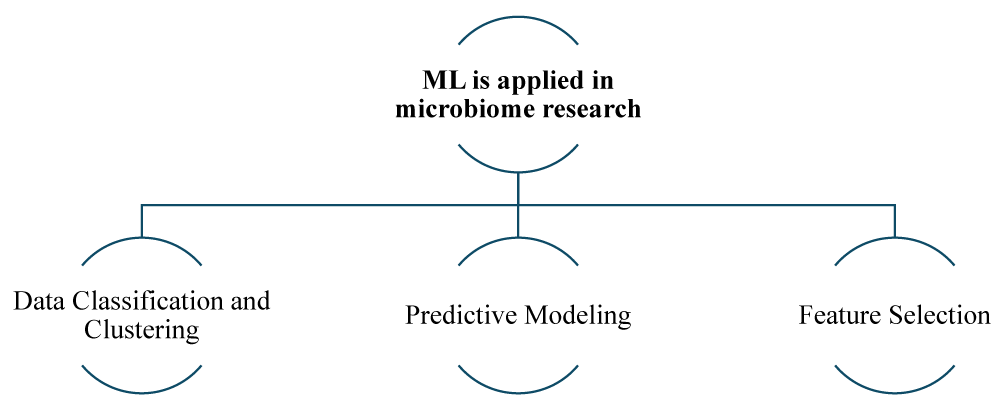
Machine Learning (ML) in microbiome research: Machine Learning involves algorithms that can learn from data and make predictions based on given data. In microbiome research, ML plays an important role in several key areas [22] (Figures 3,4).
Figure 3: Exploring AI Techniques in Microbiome-Gut-Brain Axis Research.
Figure 4: Overview of Machine Learning (ML) in Microbiome Research.
Data classification and clustering: One important application is data classification and clustering. ML Algorithms such as k-means clustering and support vector machines (SVM), etc, are used to classify and cluster microbiome data, allowing researchers to categorize different types of gut bacteria and also help in identifying patterns associated with various mental health conditions. This classification helps in understanding how specific bacterial communities correlate with different health and disease states, providing a clearer picture of the relationship between microbiome and overall well-being [23].
Predictive modeling: ML algorithm models, such as random forests and gradient-boosting machines, etc, utilize complex algorithms to analyze and interpret microbiome profiles. These models are capable of making predictions regarding various outcomes based on microbiome profiles, for instance, these models can predict the likelihood of developing mental health disorders based on the composition of an individual gut microbiota. This predictive capability is incredibly valuable as it enables the development of customized and personalized treatment plans, as well as early intervention strategies, enabling more targeted and effective mental health care [24].
Feature selection: In the field of data analysis, various advanced techniques like LASSO (Least Absolute Shrinkage and Selection Operator) [25] and principal component analysis (PCA) [26] are used to identify key features within large datasets. By utilizing these methods, researchers can identify the most important variables, allowing them to focus on specific bacterial strains and metabolic pathways that have significant relevance to mental health. This targeted approach enhances the efficiency and effectiveness of microbiome research, guiding scientists toward the most promising areas of investigation [27].
Deep Learning (DL) in microbiome research: Deep Learning, a subset of ML, uses neural networks with multiple layers to process complex datasets. Its application in microbiome research has been transformative [27] (Figure 5).
Figure 5: Overview of Deep Learning (DL) in Microbiome Research.
Pattern recognition: One of the important applications of DL is in pattern recognition· Convolutional Neural Networks (CNNs) and Recurrent Neural Networks (RNNs) are advanced machine learning techniques that are capable of recognizing complex patterns within microbiome data, which may not be easily detectable using traditional methods. By using these powerful neural networks, researchers can recognize subtle associations between gut bacteria and brain function or behaviour, leading to a more nuanced understanding of the microbiome-gut-brain axis [28,29].
Handling high-dimensional data: Utilizing deep learning models, which excel at handling high-dimensional data, particularly those generated by multi-omics methodologies including genomics, metabolomics, and proteomics, offers numerous advantages. With the capability of these models to manage and analyze large and complex datasets involved in microbiome research, integrating various types of biological data for a comprehensive analysis of the microbiome’s impact on the brain. This holistic view is essential for understanding the multifaceted nature of the microbiome-gut-brain axis and its role in mental health [28,29].
Generating hypotheses and insights: Autoencoders and generative adversarial networks (GANs) are cutting-edge technologies that analyze data to generate new hypotheses and valuable insights [31]. These models learn from existing data and create simulations, these advanced techniques can unlock previously unseen patterns and relationships. This can be particularly beneficial in the field of biomedical research, where it can lead to the identification of novel biomarkers or therapeutic targets through unprecedented exploration of complex datasets. The ability to uncover these insights from data in ways that were previously impossible has the potential to open up novel avenues for research and treatment of any medical condition. By generating hypotheses and understanding, DL models contribute to a deeper understanding of complex interactions between gut microbiota and brain health [30].
Machine Learning and Deep Learning techniques are revolutionizing microbiome-gut-brain research. By enabling advanced data analysis, these AI methods are uncovering complex interactions between gut microbiota and brain health, leading to new diagnostic and therapeutic possibilities. Their application continues to enhance our understanding of the microbiome-gut-brain axis, covering ways for innovative approaches to mental health care conditions.
Types of AI algorithms used in microbiome research
The integration of Artificial Intelligence (AI) into microbiome-gut-brain research has significantly advanced our understanding of the intricate interactions within this axis. AI algorithms offer unique strengths in processing, classifying, and predicting biological patterns and outcomes, making them invaluable tools in this field. Here, we explore the application and advantages of various AI algorithms used in microbiome research (Figure 6).
Figure 6: Overview of Types of AI Algorithms Used in Microbiome Research.
Random Forest (RF): Random Forests is a powerful collective learning method that works by creating a multitude of decision trees and then merging their results to produce an enhanced and more reliable prediction. This method is particularly beneficial in the field of microbiome research, where it can be utilized to effectively classify various microbial communities and predict mental health outcomes, based on the compositions of gut microbiota. The main strength of Random Forest is the ability to handle high-dimensional data efficiently. Additionally, it can provide estimates of feature importance, which are invaluable for identifying important microbial species associated with specific individual health conditions [32,33].
Support Vector Machines (SVM): SVM, which stands for Support Vector Machines, is a supervised learning algorithm commonly used for classification and regression-related works. It works by finding an optimal hyperplane that can greatest separate different classes of datasets with maximum margin. In microbiome analysis, where SVMS can be used to classify different types of bacteria associated with different mental health states and predict the presence of mental health disorders based on microbiome profiles. The strengths of SVM lie in its effectiveness in high-dimensional spaces, making it especially useful for tasks involving complex and multi-dimensional data. Furthermore, SVM is also known to perform well even with small to medium-sized datasets, making it a versatile tool for various applications [34,35].
K-mean clustering: K-means clustering is an unsupervised learning algorithm that aims to separate a set of data into K cluster groups based on feature similarity. This algorithm is commonly used to group similar microbiome profiles, which can help identify common microbial patterns in individuals with similar mental health conditions. K-means clustering is simple to implement and highly efficient, especially when dealing with large complex datasets, making it ideal for exploratory data analysis tasks where the goal is to gain insights from data and identify patterns or groupings [36,37].
Convolutional Neural Networks (CNN): Neural networks known as convolutional neural networks (CNNs) are the type of deep learning model that is highly effective in processing data structures that are in grid-like form, making them particularly well-suited for tasks involving images and spatial data. These specialized computer algorithms, known as convolutional neural networks, are created to adaptively learn and understand patterns in data without human interference. In microbiome research, CNNs can be used to study spatial relationships and visual patterns within microbiome composition data. They excel at capturing local patterns and hierarchical structures, making them suitable for high-resolution microbiome data [38,39].
Recurrent Neural Networks (RNN): Recurrent Neural Networks (RNNs) are a type of artificial neural network designed for sequential data processing steps. Unlike other traditional feedforward neural networks, RNNs have connections that can form directed cycles, enabling them to retain and utilize information from previous inputs. This memory capability makes RNNs particularly effective for tasks involving sequential data, such as natural language processing, time series analysis, and speech recognition. RNN finds applications in analyzing time-series microbiome data to understand how microbial compositions change over time and their temporal/ sequential relationship with mental health states. They are effective for the temporal/ sequential aspect of the dataset, RNNs can capture complex temporal dependencies and patterns in dynamic systems, making them ideal for longitudinal studies in microbiome research [40,41].
Gradient Boosting Machines (GBM): Gradient Boosting Machine (GBM) is an ensemble learning technique that builds models in a stage-wise fashion and generalizes them by optimizing a random differentiable loss function. It works by adding predictors to a collective in sequence, where each one corrects its predecessor. This allows the model to focus more on instances that were previously misclassified. GBM can be effectively used for predictive modeling in microbiome research, such as predicting disease outcomes based on microbiome features by analyzing the microbial composition and its potential impact on human health. GBM offers high predictive accuracy and flexibility, capable of handling a mix of different types of data including categorical and numerical features, which is essential for the heterogeneous nature of microbiome data [42].
Autoencoders: Autoencoders are a type of neural network used in unsupervised learning to learn effective representations of input datasets. They have a network that encodes input data into a hidden space and a network that decodes this hidden space representation to reconstruct input data. Autoencoders are typically used for tasks such as dimensionality reduction, feature learning, and data denoising. In microbiome data analysis, autoencoders can compress high-dimensional microbiome data, identifying key patterns, features, and underlying structures that are relevant to mental health. Autoencoders are capable of unsupervised learning, allowing them to learn representations of input data without requiring explicit labels. Additionally, autoencoders are effective for feature extraction, as they can capture most salient features of input data in learned latent space and autoencoders are also useful for data denoising, as they can learn to reconstruct clean representations of input data from noisy or corrupted samples, making them a valuable tool for handling large and complex microbiome datasets [43,44].
AI algorithms such as Random Forest, Support Vector Machines, K-means clustering, Convolutional Neural Networks, Recurrent Neural Networks, Gradient Boosting Machines, and Autoencoders are crucial in advancing microbiome-gut-brain research. By using these techniques, researchers can decode complex interactions within the microbiome-gut-brain axis, uncovering new insights into mental health and opening up novel therapeutic avenues. The application of these AI techniques continues to enhance our understanding of the microbiome’s role in mental health, paving the way for innovative approaches to mental health care.
AI-based analysis of microbiome data in mental health conditions
Artificial Intelligence (AI) has become a transformative tool in the analysis of microbiome data, particularly in understanding its implications for mental health conditions. AI techniques have proven important in deciphering the complex relationships between gut microbiota and mental health disorders such as depression, anxiety, multiple sclerosis, and autism spectrum disorder (ASD) (Figure 7).
Figure 7: AI Models in Microbiome Analysis.
Depression: AI techniques, particularly machine learning models, including Random Forest and Support Vector Machines (SVM), etc, have been employed to analyze the composition of gut microbiota in individuals suffering from depression. The robust capabilities of these AI models have enabled researchers to identify specific bacterial taxa that are closely associated with depressive symptoms, revealing that individuals with depression often exhibit reduced levels of beneficial bacteria and increased levels of potentially pathogenic bacteria. These findings highlight complex microbial patterns/ signatures characteristic of depression, providing valuable insights into the biological mechanisms of this mental health condition [45-47].
The implications of these AI-based findings are significant, providing a deeper understanding of the gut microbiome’s role in depression, and potentially a way for the development of targeted therapies may inform the formulation of microbiome-targeted interventions, such as probiotics or dietary approaches, aimed at restoring a healthy gut microbiome to alleviate depressive symptoms [48].
Anxiety: Recurrent Neural Networks (RNNs), a subset of neural networks designed for sequential data, play a significant role in the analysis of time-series data related to changes in the microbiome and their association with levels of anxiety. AI models have identified connections between symptoms of anxiety and specific gut microbiota profiles, such as decreased microbial diversity and the presence of certain microbial metabolites that influence the gut-brain axis. These insights into microbial factors contributing to anxiety can guide the exploration of microbiome-based treatments to mitigate anxiety symptoms. By understanding how changes in gut microbiota composition affect anxiety, researchers can develop interventions that target these specific microbial imbalances related to symptoms of anxiety [49-51].
Multiple Sclerosis (MS): Convolutional Neural Networks (CNNs) and other deep learning models have been applied to analyze the composition of gut microbiota in patients with Multiple Sclerosis (MS), taking into account the complexity and high dimensional nature of microbiome data [52,53]. AI-driven studies have revealed unique and identifiable microbiome signatures/ patterns in MS patients, these signatures/patterns are characterized by an increased presence of pro-inflammatory bacteria and a decreased presence of anti-inflammatory species within the gut microbiota of MS patients. These findings suggest potential microbial targets for therapeutic intervention, indicating that manipulating gut microbiota could help to effectively manage MS symptoms and progression. By identifying specific microbial imbalances associated with MS, AI helps to develop microbiome-targeted therapies that aim to restore a healthier balance of gut bacteria [52,54,55].
Autism Spectrum Disorder (ASD): Unsupervised learning techniques, such as autoencoders, are being utilized to effectively reduce the dimensionality of microbiome data and identify key features associated with Autism Spectrum Disorder (ASD). AI analyses have indicated significant differences in gut microbiota between individuals with ASD and neurotypical controls. These differences include an imbalance of certain microbial populations and alterations in metabolic pathways. These discoveries provide potential biomarkers that could be used for early diagnosis of ASD and suggest that manipulating the microbiome could be a viable strategy for improving symptoms associated with ASD symptoms. By identifying specific microbial signatures associated with ASD, AI aids in the development of personalized treatment approaches that target these microbial imbalances for individuals with ASD [56,57].
AI-based analysis of microbiome data offers significant insights into the microbiome-gut-brain axis and its role in mental health conditions like depression, anxiety, multiple sclerosis, and autism spectrum disorder. By using advanced AI techniques such as machine learning, neural networks, and deep learning models, researchers can uncover specific microbial signatures, patterns, and correlations with mental health states. These findings not only enhance our understanding of mechanisms but also provide a way for innovative, microbiome-targeted therapeutic strategies, etc. AI continues to be an important tool in advancing microbiome research, ultimately contributing to improved mental health outcomes for individuals [58,59].
Case studies
Case study 1: Cheng and Joe (2023) (volume 2649) explored microbiome-based machine learning for phenotypic classification. They focused on using advanced computational approaches to analyze microbiome data and improve phenotypic classification for personalized medicine and healthcare applications. Their result findings emphasize the potential of machine learning to handle and interpret large amounts of data generated in microbiome studies. The incorporation of artificial intelligence in this context not only enhances our understanding of the microbiome’s significance in both health and disease but also opens new opportunities for diagnostic and therapeutic opportunities. This case study exemplifies the transformative influence of machine learning on biological data analysis and its essential contribution to modern medical research and practice [60].
Case study 2: Xu, et al. (2023) introduced an innovative approach to cancer diagnosis using microbiome-based models enhanced by artificial intelligence. The study, published in Briefings in Bioinformatics describes the development of DeepMicroCancer, a diagnosis model that uses random forest algorithms to achieve high performance across more than twenty types of cancer tissue samples. To address the challenge of limited sample sizes for certain cancer types, the model incorporates transfer learning techniques. DeepMicroCancer demonstrated high diagnostic accuracy for both tissue and blood samples, making its flexibility and potential for clinical application. The study found that using advanced AI techniques to identify specific microbial signatures can differentiate between cancerous and healthy states, suggesting a profound interplay between microbiome and cancer. Overall, DeepMicroCancer represents a significant advancement in cancer diagnostics, it offers a reliable tool for accurate and adaptable cancer detection based on microbiome analysis [61].
The microbiome-gut-brain axis is a vital connection between the gastrointestinal tract in addition with the central nervous system this bidirectional system operates through neural, endocrine, immune, and metabolic pathways, playing an important role in maintaining overall health and significantly impacting mental health. Artificial Intelligence (AI) is being used to analyze and integrate large complex microbiome-gut-brain axis data and reveal complex relationships within this axis, offering the development of predictive modeling, biomarker discovery, and a deeper understanding of the mechanisms connecting gut microbiota to brain function. The use of AI in this field has already provided significant insights. AI models have been able to identify specific microbial signatures/ patterns associated with mental health conditions like depression, anxiety, multiple sclerosis (MS), and autism spectrum disorder (ASD). These findings highlight the potential for microbiome-targeted therapies, including dietary adjustments and probiotics, offering new possibilities for personalized treatment approaches. The integration of AI in microbiome research shows promising therapeutic applications. AI-driven insights can guide the development of novel interventions aimed at restoring healthy gut microbiota and alleviating symptoms of mental health disorders. However, several challenges remain, including the need for standardized data collection, processing methods to ensure consistency and reliability, and ethical considerations around data privacy and AI use in healthcare.
In conclusion, artificial intelligence’s (AI) role in unraveling the complexities of the microbiome-gut-brain axis cannot be overstated. By providing deeper insights into complex and dynamic relationships between gut microbiota and brain function. This invaluable contribution is a way for the development of revolutionary therapeutic strategies. As ongoing research in this domain continues, the integration of AI promises to unlock new frontiers in mental health care, offering hope for more effective and personalized treatments.
Although AI-generated tools were used to generate this eBook/Article, the concepts and central ideas it contains were entirely original and devised by a human writer. The AI merely assisted in the writing process, but the creative vision and intellectual property belong to the human author.
- Rutsch A, Kantsjö JB, Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol. 2020 Dec 10;11:604179. doi: 10.3389/fimmu.2020.604179. PMID: 33362788; PMCID: PMC7758428.
- LaGreca M, Hutchinson DR, Skehan L. The microbiome and neurotransmitter activity. J Sci Med. 2022;3(2). https://doi.org/10.37714/JOSAM.V3I2.90
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015 Apr-Jun;28(2):203-209. PMID: 25830558; PMCID: PMC4367209.
- Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018 Feb 7;12:49. doi: 10.3389/fnins.2018.00049. PMID: 29467611; PMCID: PMC5808284.
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011 Jul 13;12(8):453-66. doi: 10.1038/nrn3071. PMID: 21750565; PMCID: PMC3845678.
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front Neuroendocrinol. 2018 Oct;51:80-101. doi: 10.1016/j.yfrne.2018.04.002. Epub 2018 May 16. PMID: 29753796.
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017 Feb;20(2):145-155. doi: 10.1038/nn.4476. Epub 2017 Jan 16. PMID: 28092661; PMCID: PMC6960010.
- Sittipo P, Choi J, Lee S, Lee YK. The function of gut microbiota in immune-related neurological disorders: a review. J Neuroinflammation. 2022 Jun 15;19(1):154. doi: 10.1186/s12974-022-02510-1. PMID: 35706008; PMCID: PMC9199126.
- Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135-157. doi: 10.1080/19490976.2019.1638722. Epub 2019 Aug 1. PMID: 31368397; PMCID: PMC7053956.
- Gong Y, Chen A, Zhang G, Shen Q, Zou L, Li J, Miao YB, Liu W. Cracking Brain Diseases from Gut Microbes-Mediated Metabolites for Precise Treatment. Int J Biol Sci. 2023 Jun 4;19(10):2974-2998. doi: 10.7150/ijbs.85259. PMID: 37416776; PMCID: PMC10321288.
- Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J (Engl). 2016 Oct 5;129(19):2373-80. doi: 10.4103/0366-6999.190667. PMID: 27647198; PMCID: PMC5040025.
- Graham S, Depp C, Lee EE, Nebeker C, Tu X, Kim HC, Jeste DV. Artificial Intelligence for Mental Health and Mental Illnesses: an Overview. Curr Psychiatry Rep. 2019 Nov 7;21(11):116. doi: 10.1007/s11920-019-1094-0. PMID: 31701320; PMCID: PMC7274446.
- Zeng T, Yu X, Chen Z. Applying artificial intelligence in the microbiome for gastrointestinal diseases: A review. J Gastroenterol Hepatol. 2021 Apr;36(4):832-840. doi: 10.1111/jgh.15503. PMID: 33880762.
- Goh KK, Liu YW, Kuo PH, Chung YE, Lu ML, Chen CH. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019 Dec;282:112568. doi: 10.1016/j.psychres.2019.112568. Epub 2019 Sep 17. PMID: 31563280.
- Amato KR, Arrieta MC, Azad MB, Bailey MT, Broussard JL, Bruggeling CE, Claud EC, Costello EK, Davenport ER, Dutilh BE, Swain Ewald HA, Ewald P, Hanlon EC, Julion W, Keshavarzian A, Maurice CF, Miller GE, Preidis GA, Segurel L, Singer B, Subramanian S, Zhao L, Kuzawa CW. The human gut microbiome and health inequities. Proc Natl Acad Sci U S A. 2021 Jun 22;118(25):e2017947118. doi: 10.1073/pnas.2017947118. PMID: 34161260; PMCID: PMC8237592.
- Zhang VR, Ramachandran GK, Loo EXL, Soh AYS, Yong WP, Siah KTH. Volatile organic compounds as potential biomarkers of irritable bowel syndrome: A systematic review. Neurogastroenterol Motil. 2023 Jul;35(7):e14536. doi: 10.1111/nmo.14536. Epub 2023 Feb 13. PMID: 36780514.
- Breiteneder H, Peng YQ, Agache I, Diamant Z, Eiwegger T, Fokkens WJ, Traidl-Hoffmann C, Nadeau K, O'Hehir RE, O'Mahony L, Pfaar O, Torres MJ, Wang DY, Zhang L, Akdis CA. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020 Dec;75(12):3039-3068. doi: 10.1111/all.14582. Epub 2020 Sep 30. PMID: 32893900; PMCID: PMC7756301.
- Foster JA. Modulating brain function with microbiota. Science. 2022 May 27;376(6596):936-937. doi: 10.1126/science.abo4220. Epub 2022 May 26. PMID: 35617384.
- Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020 Jan 31;11:25. doi: 10.3389/fendo.2020.00025. PMID: 32082260; PMCID: PMC7005631.
- Jiang Y, Luo J, Huang D, Liu Y, Li DD. Machine Learning Advances in Microbiology: A Review of Methods and Applications. Front Microbiol. 2022 May 26;13:925454. doi: 10.3389/fmicb.2022.925454. PMID: 35711777; PMCID: PMC9196628.
- Meystre S, van Stiphout R, Goris A, Gaitan S. AI-Based Gut-Brain Axis Digital Twins. Stud Health Technol Inform. 2023 May 18;302:1007-1008. doi: 10.3233/SHTI230327. PMID: 37203554.
- Hernández Medina R, Kutuzova S, Nor Nielsen K, Johansen J, Hestbjerg Hansen L, Nielsen M, Rasmussen S. Machine learning and deep learning applications in microbiome research. ISME Commun. 2022 Dec;2(1):98. https://doi.org/10.1038/s43705-022-00182-9
- Hayes CL, Peters BJ, Foster JA. Microbes and mental health: Can the microbiome help explain clinical heterogeneity in psychiatry? Front Neuroendocrinol. 2020 Jul;58:100849. doi: 10.1016/j.yfrne.2020.100849. Epub 2020 Jun 1. PMID: 32497560.
- Shen D, Liu C, Xu R, Zhang F. Human gut microbiota: dysbiosis and manipulation. Front Cell Infect Microbiol. 2012 Sep 27;2:123. doi: 10.3389/fcimb.2012.00123. Retraction in: Front Cell Infect Microbiol. 2013 Dec 19;3:104. doi: 10.3389/fcimb.2013.00104. PMID: 23061053; PMCID: PMC3459033.
- Lu F, Petkova E. A comparative study of variable selection methods in the context of developing psychiatric screening instruments. Stat Med. 2014 Feb 10;33(3):401-21. doi: 10.1002/sim.5937. Epub 2013 Aug 11. PMID: 23934941; PMCID: PMC4026268.
- Cho G, Yim J, Choi Y, Ko J, Lee SH. Review of Machine Learning Algorithms for Diagnosing Mental Illness. Psychiatry Investig. 2019 Apr;16(4):262-269. doi: 10.30773/pi.2018.12.21.2. Epub 2019 Apr 8. PMID: 30947496; PMCID: PMC6504772.
- Rani P, Kotwal S, Manhas J, Sharma V, Sharma S. Machine Learning and Deep Learning Based Computational Approaches in Automatic Microorganisms Image Recognition: Methodologies, Challenges, and Developments. Arch Comput Methods Eng. 2022;29(3):1801-1837. doi: 10.1007/s11831-021-09639-x. Epub 2021 Aug 31. PMID: 34483651; PMCID: PMC8405717.
- Grapov D, Fahrmann J, Wanichthanarak K, Khoomrung S. Rise of Deep Learning for Genomic, Proteomic, and Metabolomic Data Integration in Precision Medicine. OMICS. 2018 Oct;22(10):630-636. doi: 10.1089/omi.2018.0097. Epub 2018 Aug 20. PMID: 30124358; PMCID: PMC6207407.
- Fiannaca A, La Paglia L, La Rosa M, Lo Bosco G, Renda G, Rizzo R, Gaglio S, Urso A. Deep learning models for bacteria taxonomic classification of metagenomic data. BMC Bioinformatics. 2018 Jul 9;19(Suppl 7):198. doi: 10.1186/s12859-018-2182-6. PMID: 30066629; PMCID: PMC6069770.
- Zieliński B, Plichta A, Misztal K, Spurek P, Brzychczy-Włoch M, Ochońska D. Deep learning approach to bacterial colony classification. PLoS One. 2017 Sep 14;12(9):e0184554. doi: 10.1371/journal.pone.0184554. PMID: 28910352; PMCID: PMC5599001.
- Ramezanian Panahi M, Abrevaya G, Gagnon-Audet J-C, Voleti V, Rish I, Dumas G. Generative models of brain dynamics: A review. Quant Biol Neurons Cogn. 2021. https://doi.org/10.48550/arXiv.2112.12147
- Chen X, Ishwaran H. Random forests for genomic data analysis. Genomics. 2012 Jun;99(6):323-9. doi: 10.1016/j.ygeno.2012.04.003. Epub 2012 Apr 21. PMID: 22546560; PMCID: PMC3387489.
- Zhang L, Wang Y, Chen J, Chen J. RFtest: A Robust and Flexible Community-Level Test for Microbiome Data Powerfully Detects Phylogenetically Clustered Signals. Front Genet. 2022 Jan 24;12:749573. doi: 10.3389/fgene.2021.749573. PMID: 35140735; PMCID: PMC8819960.
- Topçuoğlu BD, Lesniak NA, Ruffin MT 4th, Wiens J, Schloss PD. A Framework for Effective Application of Machine Learning to Microbiome-Based Classification Problems. mBio. 2020 Jun 9;11(3):e00434-20. doi: 10.1128/mBio.00434-20. PMID: 32518182; PMCID: PMC7373189.
- Awad M, Khanna R. Support Vector Machines for Classification. In: Efficient Learning Machines. Berkeley, CA: Apress; 2015. https://doi.org/10.1007/978-1-4302-5990-9_3
- Celebi ME, Kingravi HA, Vela PA. A comparative study of efficient initialization methods for the k-means clustering algorithm. Expert Syst Appl. 2013;40(1):200-210. https://doi.org/10.1016/j.eswa.2012.07.021
- Yao YK, Liu Y, Li Z, Chen XY. An Effective K-Means Clustering Based SVM Algorithm. Appl Mech Mater. 2013;333-335:1344-1348. https://doi.org/10.4028/www.scientific.net/AMM.333-335.1344
- Gu J, Wang Z, Kuen J, Ma L, Shahroudy A, Shuai B, Liu T, Wang X, Wang G. Recent Advances in Convolutional Neural Networks. Computer Science, Computer Vision and Pattern Recognition, Neural and Evolutionary Computing. 2017. https://doi.org/10.48550/arXiv.1512.07108
- Hsu T, Epting WK, Kim H, et al. Microstructure Generation via Generative Adversarial Network for Heterogeneous, Topologically Complex 3D Materials. JOM. 2021;73:90-102. https://doi.org/10.1007/s11837-020-04484-y
- Furusawa C, Tanabe K, Ishii C, Kagata N, Tomita M, Fukuda S. Decoding gut microbiota by imaging analysis of fecal samples. iScience. 2021 Nov 22;24(12):103481. doi: 10.1016/j.isci.2021.103481. PMID: 34927025; PMCID: PMC8652011.
- Graves, A. (2014). Generating sequences with recurrent neural networks. arXiv, 1308(0850v5). https://doi.org/10.48550/arXiv.1308.0850
- Hilton CB, Milinovich A, Felix C, Vakharia N, Crone T, Donovan C, Proctor A, Nazha A. Personalized predictions of patient outcomes during and after hospitalization using artificial intelligence. NPJ Digit Med. 2020 Apr 3;3:51. doi: 10.1038/s41746-020-0249-z. PMID: 32285012; PMCID: PMC7125114.
- Charte D, Charte F, García S, del Jesus MJ, Herrera F. A practical tutorial on autoencoders for nonlinear feature fusion: Taxonomy, models, software and guidelines. Inf Fusion. 2018;44:78-96. https://doi.org/10.1016/j.inffus.2017.12.007
- Bank D, Koenigstein N, Giryes R. Autoencoders. arXiv. 2021;2003(05991v2). https://doi.org/10.48550/arXiv.2003.05991
- Ermers NJ, Hagoort K, Scheepers FE. The Predictive Validity of Machine Learning Models in the Classification and Treatment of Major Depressive Disorder: State of the Art and Future Directions. Front Psychiatry. 2020 May 25;11:472. doi: 10.3389/fpsyt.2020.00472. PMID: 32523557; PMCID: PMC7261928.
- Mali D, Kumawat K, Kumawat G, Chakrabarti P, Poddar S, Chakrabarti T, Hussaine J, Kamali A-M, Bolsev V, Kateb B, Nami M. A Machine Learning Technique to Analyze Depressive Disorder. Res Square. 2021. DOI: https://doi.org/10.21203/rs.3.rs-322564/v1.
- Tran BX, McIntyre RS, Latkin CA, Phan HT, Vu GT, Nguyen HLT, Gwee KK, Ho CSH, Ho RCM. The Current Research Landscape on the Artificial Intelligence Application in the Management of Depressive Disorders: A Bibliometric Analysis. Int J Environ Res Public Health. 2019 Jun 18;16(12):2150. doi: 10.3390/ijerph16122150. PMID: 31216619; PMCID: PMC6617113.
- Gao S, Calhoun VD, Sui J. Machine learning in major depression: From classification to treatment outcome prediction. CNS Neurosci Ther. 2018 Nov;24(11):1037-1052. doi: 10.1111/cns.13048. Epub 2018 Aug 23. PMID: 30136381; PMCID: PMC6324186.
- Bravo JA, Julio-Pieper M, Forsythe P, Kunze W, Dinan TG, Bienenstock J, Cryan JF. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012 Dec;12(6):667-72. doi: 10.1016/j.coph.2012.09.010. Epub 2012 Oct 4. PMID: 23041079.
- Butler MI, Bastiaanssen TFS, Long-Smith C, Morkl S, Berding K, Ritz NL, Strain C, Patangia D, Patel S, Stanton C, O'Mahony SM, Cryan JF, Clarke G, Dinan TG. The gut microbiome in social anxiety disorder: evidence of altered composition and function. Transl Psychiatry. 2023 Mar 20;13(1):95. doi: 10.1038/s41398-023-02325-5. PMID: 36941248; PMCID: PMC10027687.
- Kumar A, Pramanik J, Goyal N, Chauhan D, Sivamaruthi BS, Prajapati BG, Chaiyasut C. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals (Basel). 2023 Apr 9;16(4):565. doi: 10.3390/ph16040565. PMID: 37111321; PMCID: PMC10146621.
- Mestre L, Carrillo-Salinas FJ, Mecha M, Feliú A, Espejo C, Álvarez-Cermeño JC, Villar LM, Guaza C. Manipulation of Gut Microbiota Influences Immune Responses, Axon Preservation, and Motor Disability in a Model of Progressive Multiple Sclerosis. Front Immunol. 2019 Jun 14;10:1374. doi: 10.3389/fimmu.2019.01374. PMID: 31258540; PMCID: PMC6587398.
- Bronzini M, Maglione A, Rosso R, Matta M, Masuzzo F, Rolla S, Clerico M. Feeding the gut microbiome: impact on multiple sclerosis. Front Immunol. 2023 May 25;14:1176016. doi: 10.3389/fimmu.2023.1176016. PMID: 37304278; PMCID: PMC10248010.
- Galluzzo P, Capri FC, Vecchioni L, Realmuto S, Scalisi L, Cottone S, Nuzzo D, Alduina R. Comparison of the Intestinal Microbiome of Italian Patients with Multiple Sclerosis and Their Household Relatives. Life (Basel). 2021 Jun 26;11(7):620. doi: 10.3390/life11070620. PMID: 34206853; PMCID: PMC8307959.
- Hasic Telalovic J, Music A. Using data science for medical decision making case: role of gut microbiome in multiple sclerosis. BMC Med Inform Decis Mak. 2020 Oct 12;20(1):262. doi: 10.1186/s12911-020-01263-2. PMID: 33046051; PMCID: PMC7549194.
- Morton JT, Jin DM, Mills RH, Shao Y, Rahman G, McDonald D, Zhu Q, Balaban M, Jiang Y, Cantrell K, Gonzalez A, Carmel J, Frankiensztajn LM, Martin-Brevet S, Berding K, Needham BD, Zurita MF, David M, Averina OV, Kovtun AS, Noto A, Mussap M, Wang M, Frank DN, Li E, Zhou W, Fanos V, Danilenko VN, Wall DP, Cárdenas P, Baldeón ME, Jacquemont S, Koren O, Elliott E, Xavier RJ, Mazmanian SK, Knight R, Gilbert JA, Donovan SM, Lawley TD, Carpenter B, Bonneau R, Taroncher-Oldenburg G. Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat Neurosci. 2023 Jul;26(7):1208-1217. doi: 10.1038/s41593-023-01361-0. Epub 2023 Jun 26. PMID: 37365313; PMCID: PMC10322709.
- Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci. 2017 Apr 28;11:120. doi: 10.3389/fncel.2017.00120. PMID: 28503135; PMCID: PMC5408485.
- Zhang F, Wei Y, Liu J, Wang Y, Xi W, Pan Y. Identification of Autism spectrum disorder based on novel feature selection method and Variational Autoencoder. Comput Biol Med. 2022;148:105854. https://doi.org/10.1016/j.compbiomed.2022.105854
- Yin Z, Ding X, Zhang X, Wu Z, Wang L, Xu X, Li G. Early Autism Diagnosis based on Path Signature and Siamese Unsupervised Feature Compressor. arXiv. 2024:2307.06472 [cs.CV]. Available from: https://arxiv.org/abs/2307.06472
- Cheng X, Joe B. Artificial Intelligence in Medicine: Microbiome-Based Machine Learning for Phenotypic Classification. Methods Mol Biol. 2023;2649:281-288. doi: 10.1007/978-1-0716-3072-3_14. PMID: 37258868.
- Xu W, Wang T, Wang N, Zhang H, Zha Y, Ji L, Chu Y, Ning K. Artificial intelligence-enabled microbiome-based diagnosis models for a broad spectrum of cancer types. Brief Bioinform. 2023;24(3). https://doi.org/10.1093/bib/bbad178