More Information
Submitted: November 11, 2023 | Approved: November 30, 2023 | Published: December 01, 2023
How to cite this article: Davies V, Heaman A, Brereton J. A Gecko-eye View of Naturalistic Enclosures. Insights Biol Med. 2023; 7: 013-019.
DOI: 10.29328/journal.ibm.1001026
Copyright License: © 2023 Davies V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Lygodactylus williamsi; Reptile behaviour; Visitor perception; Naturalism; Enclosure design
A Gecko-eye View of Naturalistic Enclosures
Victoria Davies, Abigail Heaman and James Brereton*
University Centre Sparsholt, Westley Lane, Sparsholt, Winchester, Hampshire, SO21 2NF, UK
*Address for Correspondence: James Brereton, University Centre Sparsholt, Westley Lane, Sparsholt, Winchester, Hampshire, SO21 2NF, UK, Email: [email protected]
Naturalistic enclosures have become a popular exhibition technique for zoos, and reptiles and amphibians are regularly housed in these exhibits. While a considerable sum of research indicates that visitors prefer naturalistic exhibits, there are fewer studies documenting the behaviour and welfare of animals housed under these conditions. This study investigated the impact of a naturalistic enclosure on the behaviour of the turquoise dwarf gecko (Lygodactylus williamsi), and the welfare perceptions of visitors. When kept under naturalistic enclosures, dwarf geckos were seen to bask (p = 0.022), and engage in inactive behaviours (p = 0.001) significantly less frequently. A non-significant decrease was also seen in locomotion | (p = 0.074). While time spent hidden remains a confounding factor for behavioural analysis, the study indicates that when provided with hiding opportunities, L. williamsi may spend a considerable amount of time hidden from the public. Questionnaire analysis revealed that 84.6% of individuals believed that naturalistic enclosures demonstrated better welfare. Additionally, individuals who had previously owned a reptile were more likely to identify that areas to hide, enrich, and mimic the natural environment were important aspects of enclosure design. While the actual benefits of naturalistic enclosure design cannot be fully addressed by this study, this work suggests that visitors tend to inherently believe that naturalistic enclosures facilitate better welfare, even if they are not aware of the natural environment of the species being housed. This requires keepers to consider both aspects of functionality and enclosure relevance when designing exhibits for herptiles.
Considerable research exists on the topic of enclosure design for a wide range of zoo taxa including primates [1], felids [2], and ursids [3]. While there is a plethora of enclosure design studies for mammalian species [4], comparatively fewer studies have been initiated for reptile or amphibian species, collectively referred to as herptiles. The International Union for Conservation of Nature [5] has named 6,278 reptiles and 6,609 amphibian species, of which many are housed in captive collections. By contrast, the IUCN [5] has named only 5,674 mammal species, though these are disproportionately better studied [6]. Further research is necessary to better understand the environmental and enclosure needs of herptiles, so that enclosure relevance may be evidence-based in the future [6,7].
Enclosure naturalism has been a consistently popular area of study in the field of zoo biology [8-10]. Enclosure naturalism is defined as the provision of natural features, such as flora and fauna, in an animal’s enclosure [9]. Andersen [8] suggests that these ‘naturalistic’ features should mirror a species’ natural habitat. Naturalistic enclosures and husbandry styles have become increasingly popular in zoos, and many novel naturalistic exhibits have been produced [12]. However, zoo animal exhibits have a dual purpose; they must be of interest to visitors and have function for the animals that live within them [13]. Studies, therefore, that address the visitor perspective, and the functional use of an enclosure to animals, are to be welcomed [14].
Visitor perspectives on exhibit naturalism
Studies by Moss and Esson [15] and Moss, et al. [14] indicate that zoo visitors prefer to spend time in enclosures containing animals that are large, active, and easily visible. A naturalistic exhibit, however, may contain considerable foliage that might block the visitor’s view of an animal (Davey, 2007). Visibility of animals is intrinsically linked with visitor satisfaction and may result in frustration if visibility is impaired [16]. Despite this, zoo visitor research suggests that individuals may spend longer viewing naturalistic enclosures [17,18]. Davey’s [9] study also suggests that visitors may interact with naturalistic enclosures more, and engage in more animal-related conversations while viewing these exhibits. Though animal visibility may often be reduced, it appears in the literature that naturalistic exhibits are favourable to zoo visitors. This may be because naturalistic exhibits are often more complex and visually appealing [10].
Visitors also believe that naturalistic exhibits are beneficial for their zoo animal inhabitants. In their study on tiger enclosures, Melfi, et al. [19] identified that visitors believed the most naturalistic (greenest) exhibits were best for tiger welfare. Indeed, Fàbregas, et al. [10] identified that among Spanish zoos, a naturalistic enclosure equated to better welfare for animals. Visitors, therefore, may be in favour of seeing more naturalistic enclosures in future zoos.
Naturalistic enclosures also provide an educational function to visitors [20,21]. Visitors may be unaware of the original habitat of many zoo species; a well-designed exhibit may therefore help to inform visitors about a species’ natural environment. For example, there is a common misconception among many visitors that penguins are found only in cold environments [22]. Carefully planned exhibit design, alongside signage, may facilitate visitors learning that species such as the Humboldt penguin (Spehniscus humboldti) are found in the warmer climates of Chile and Peru.
Animal research on exhibit naturalism
Throughout Europe and America, modern zoos rarely take animals from the wild [23]. With the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) effective from, 1975, trips to collect wild animals have become rare [24]. With the exception of some long-lived taxa such as tortoises, parrots, and crocodiles, most zoo animals have been reared in captivity, and therefore have no knowledge of their wild habitat.
Many zoo researchers suggest that an enclosure functionality approach is a more relevant way of addressing exhibit design [25]. For example, a captive-born sloth (Choloepus didactylus) possesses no inherited knowledge of its ancestral rainforests. However, sloths have evolved over millions of years to live upside down, and their behaviour and physiology reflect this. While the branches themselves may not be essential for good sloth welfare, opportunities to rest and climb upside down may be. Alternatives to branches, such as rope ladders and poles, may be equally relevant for welfare, provided they meet the same functions as the branches.
Fàbregas, et al. [10] comprehensive comparative study of zoo exhibits revealed that naturalistic enclosures provided better welfare for their inhabitants more frequently than artificial enclosures did. Behavioural research on naturalistic exhibits for orangutans (Pongo pygmaeus) indicated that naturalistic enclosures reduced the impact of high visitor numbers [20]. The increased enclosure complexity allowed orangutans to engage in a greater diversity of behaviours, and to avoid visitors during peak times.
Herptiles provide unique opportunities for behavioural studies to exhibit naturalism [26] and enrichment [27]. Many small herptile species, such as geckos, mantellas, and dart frogs, are commonly kept in naturalistic vivariums [28]. This may facilitate education, allowing zoos to create displays that resemble a species’ natural environment, and potentially encouraging support for in situ conservation measures [29].
While naturalistic exhibits are frequently utilised, there are relatively few studies on the benefits of these enclosures to their inhabitants [4,25]. Studies on herptile and invertebrate welfare are confounded in that few measures of positive or negative welfare have been identified [23,30,31]. Those measures that do exist also require validation for individual species. For example, Moszuti, et al. [32] suggested that neck extension could be used to assess welfare and anxiety in red-footed tortoises (Chelonoidis carbonaria). However, a similar measure of welfare was not identified for the bearded dragon (Pogona vitticeps) [32]. For many herptile species, natural activity budgets are not known [25]. An increased or decreased level of inactivity, therefore, should not be used as an indicator of welfare, unless species-specific comparisons are available [28]. To validate measures of welfare, Bashaw, et al. [27] used behavioural diversity and abnormal repetitive behaviours for leopard geckos (Eublepharis macularius) when provided with enrichment. Behavioural diversity may be valuable for assessing welfare, though consideration should be given to the fact that not all behaviours are beneficial for welfare.
Turquoise dwarf gecko
The turquoise dwarf gecko (Lygodactylus williamsi), also known by its trade name of ‘electric blue day gecko’, is a small, brightly coloured gecko originating from Tanzania [33,34]. Famed for its bright colouration, wild gecko populations have decreased as a result of collection for the pet trade [35], and the species is now listed as Critically Endangered by the IUCN [36,37]. L. williamsi is sexually dimorphic, with males possessing bright blue scalation, and females duller brown in colour [33]. Wild blue geckos appear to be found solely on the screwpine (Pandanus rabaiensis); on a large screwpine, several geckos may establish territories [36,38]. The bright colouration of males and predictable placement, along with their restricted Tanzanian range, further predispose L. williamsi to extinction [33].
Given their important conservation education message on the impact of the pet trade, and the need to produce a captive-bred reserve population in case of wild extirpation, the turquoise dwarf gecko is an excellent candidate for zoo breeding programmes [33,39]. Research projects and student practical sessions may also be used to raise awareness of the plight of this gecko species.
This research project incorporated both a behavioural study on geckos and a questionnaire component. Questionnaires were used to ascertain people’s perceptions of welfare: a total of 65 questionnaires were completed by students from both the Higher Education access course and Further Education courses at Sparsholt College, Hampshire. Informed consent was obtained prior to the completion of questionnaires. Data collection for both the questionnaire and behavioural observations took place between October and December 2017.
Behaviour
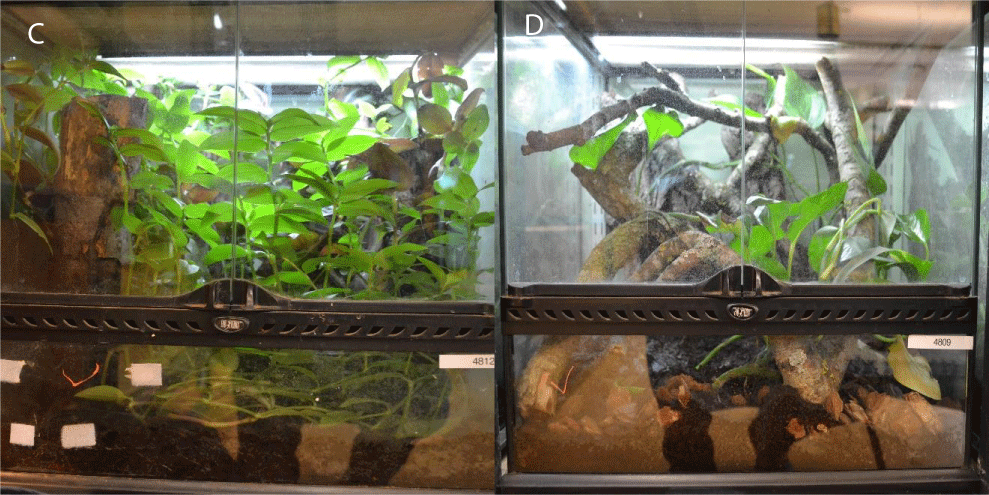
A total of 4.4 dwarf geckos were observed over a period of 36 hours. Sessions were two hours long and took place during three scheduled times of the day: morning (08:30 – 10:30), noon (12:45 – 14:45), and afternoon (14:45 – 16:45). Animals were housed in 1.1 pairs: four Exo Terra™ 60x60x60 centimetre vivariums were utilised to provide replication for the project. Vivariums A and B, described as artificial, contained no live plants but retained the branches instead for the geckos to climb upon (Figure 1). Vivariums C and D, described as naturalistic enclosures, contained a mixture of foliage and branches including devil’s ivy (Epipremnum aureum), spider plants (Chlorophytum comosum), and wandering Jew plant (Tradescantia zebrina) (Figures 1,2).
Figure 1: Enclosures A and B containing only substrate and branches, described as artificial enclosures.
Figure 2: Naturalistic enclosures C and D, containing a range of branches and live plants.
All other aspects of the four enclosures remained the same; the temperature was not changed, and the animals were fed twice per week on crickets as per their normal routine. The vivariums were arranged on two shelving units so that one naturalistic and one artificial enclosure was placed on each shelf.
For each vivarium, instantaneous scan sampling at one-minute intervals was used [40]. Continuous scan sampling was utilised in order to measure the number of event behaviours expressed by the geckos (Table 1) [40].
| Table 1: Event behaviours conducted by L. williamsi: adapted from Pandav, et al. [41]. | |
| Event Behaviour | |
| Display | Animal demonstrates head bob (head raised and lowered with no other body movement) or presses up (rapid raising and lowering of the anterior region of the body using the forelimbs). |
| Head lift | Neck is stretched at right angles to the body with the cranium (head) tipped upwards. |
| Jump | Animal springs from one place in the vivarium to another, with the entire body leaving the ground. |
| Gular extension | Skin of the throat is extended. |
| Scratch | Animal briefly scrapes the skin with its claws. |
| Tail wagging | Rear part of the animal’s body moves from side to side. |
Prior to observing the geckos an ethogram was devised (Tables 1,2). Following this, a pilot study took place and further behaviours were noted and added to the ethogram. No specific ethograms for L. williamsi were available, therefore behaviours were generated from work on reptiles by Pandav, et al. [41]. The ethogram was modified based on observations from an initial pilot study.
| Table 2: State behaviours conducted by L. williamsi: adapted from Pandav, et al. [41]. | |
| State Behaviour | Definition |
| Basking | The stationary animal is either against a heat source or directly under a heat source. |
| Eating | Animal consumes food. |
| Hidden | Animal is concealed within the enclosure. Only part of the animal is on view, not enough to judge a specific behaviour. |
| Inactive | The animal is either standing or resting on part of the enclosure or its furnishings. The animal is stationary. |
| Movement | The animal is walking, climbing, or running across the exhibit, glass, live plants, or branches. |
| Social interaction | The animal approaches another individual or engages in pursuit or fleeing behaviour. Individuals may attempt to bite or scratch another individual. |
Questionnaire data collection
To identify perceptions of welfare, students took part in the questionnaire. A series of seven questions were asked, identifying the age and gender of individuals. Questions evaluated whether individuals had previously owned a reptile, their understanding of welfare and naturalism, and the individual’s understanding of natural behaviour. The questionnaires were given out, completed, and returned during supervised sessions. The questionnaire (Appendix-1Appendix-1)consisted of multiple-choice and one-word answers to avoid any form of bias. The questionnaires were completed individually and the students had access to a picture of one artificial and one naturalistic enclosure.
Data analysis for behaviour
All raw data was collected using Microsoft Excel™ 2016, and analysed using Minitab version 18. For the analysis of behaviour, hidden behaviours were included in the analysis. Activity budgets were calculated for the artificial (A and B) and naturalistic (C and D) enclosures. Analysis was conducted on the raw data. Following testing for normality, all behaviour data was identified as being not normally distributed. Mann-Whitney U tests were conducted to compare the behaviour between the naturalistic and artificial enclosures.
The data from the questionnaires was collated using an Excel spreadsheet; from this, percentages were calculated. Owing to the nature of the qualitative results, Chi2 goodness of fit tests were chosen for the statistical analysis.
Behavioural data
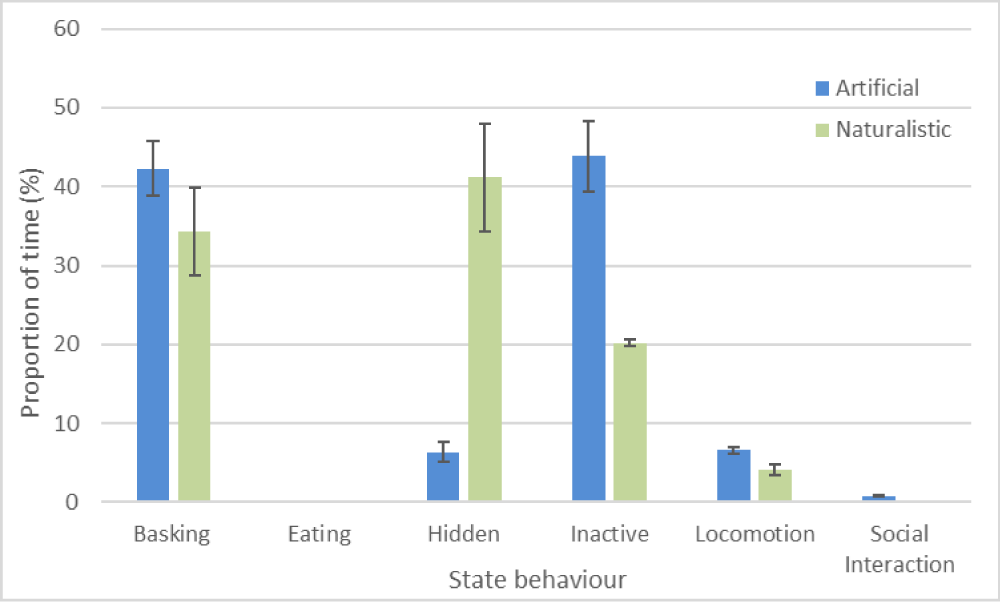
Activity budgets were generated for geckos under both naturalistic and artificial conditions (Figure 3). For state behaviours, geckos in the naturalistic enclosures appeared to spend a significantly lower proportion of time engaged in basking (W = 1437.5, n = 18, p = 0.022), inactive behaviours (W = 415, n = 18, p = 0.01), but not locomotion (W = 389, n = 18, p = 0.074). Geckos spent longer hidden when in the naturalistic enclosure, but this was not significant (W = 1122.5, n = 18, p = 0.156). Given the amount of time spent hiding, actual values for basking and inactivity could be considerably higher.
Figure 3: Activity budget for L. williamsi under naturalistic and artificial conditions (+/- standard error).
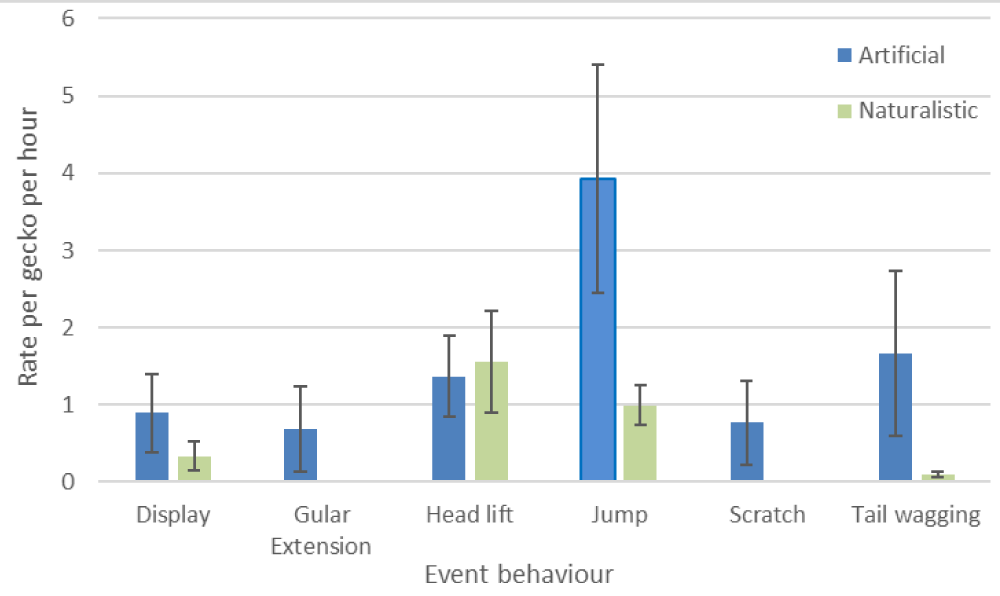
Event behaviours were calculated into an average number of events per gecko per hour (Figure 4). The proportion of time spent hidden was used as a correction factor for both the naturalistic and artificial enclosures. Statistical analyses were not conducted on the event behaviours, given the difference in visibility between the two enclosures.
Figure 4: Average number of events per gecko per hour for naturalistic and artificial enclosures (+/- standard error), corrected for periods in which the geckos were hidden.
Questionnaire responses
A total of 65 individuals filled in the questionnaire, which consisted of 57 (87.7%) females and 8 (12.3%) males. Of these individuals, 16 (24.6%) had previously kept a reptile, and 49 (75.4%) had not. Overall, 84.6% of all individuals suggested that the naturalistic enclosure was better for animal welfare, with the remaining 15.4% suggesting that the artificial enclosure was optimal.
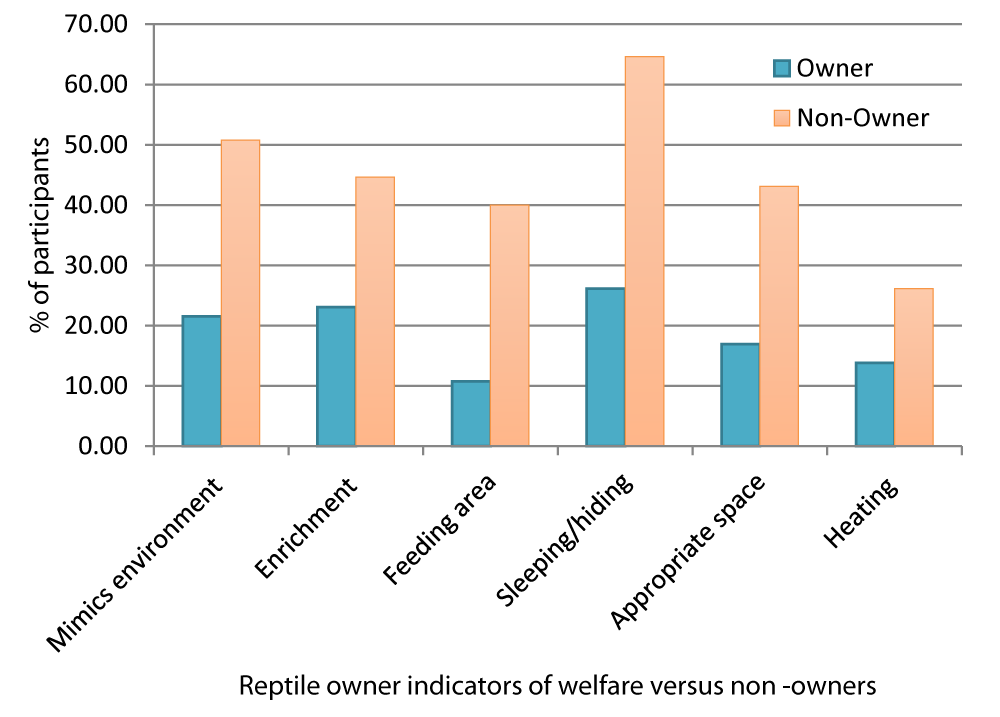
Reptile owners and non-reptile owners were compared in their perception of whether the naturalistic or artificial exhibit showed better welfare. 21.53% of reptile owners indicated that the naturalistic enclosure was better for welfare, as opposed to 63.07% from non-reptile owners.
The percentages behind the choices of the participants were calculated (Figure 5) to determine differences in perception between owners and non-owners of reptiles.
Figure 5: Percentage of welfare indicators chosen by owners versus non-owners.
Behavioural data
Previous studies have illustrated that a naturalistic exhibit may increase behavioural diversity and natural behaviours for a range of species [9]. In the current study, however, this trend was not identified for gecko state behaviours. Reduced visibility due to plant matter acts as a source of error in this dataset.
One conclusion that may be drawn from the study is that when given places to hide in an enclosure, L. williamsi will make use of this resource. While less than ideal from a visitor’s perspective [9], giving captive animals a choice is a key way of improving welfare [6]. Additionally, there is some research to suggest that visitors will spend longer viewing naturalistic exhibits; by engaging for longer, they may still be likely to view an animal [20]. Given the proportion of time spent hiding, conclusions cannot be drawn as to whether the gecko welfare was improved by naturalistic or artificial settings.
Event behaviours may be valuable indicators of an animal’s response to its environment. For example, Moszuti, et al. [32] used the prevalence of tongue flicks for bearded dragons to identify their response to a novel environment. Behavioural diversity is often used as a measure of welfare; it is often suggested that a more complex environment may allow an animal to display a wider range of natural behaviours. It had been hypothesised that a naturalistic enclosure, in this study, would allow the reptiles to showcase a greater range of behaviours.
In the current study, the prevalence of all measured event behaviours, except for head lifts, was reduced under the naturalistic setting. Additionally, the data was corrected for the amount of time that the geckos spent hidden. Given the amount of time in which the geckos were out of sight for the naturalistic exhibit, the recorded values for event behaviours cannot be treated as exact. Instead, the values should be treated only as a comparison of event behaviour prevalence when geckos choose to be visible.
It is possible that the geckos in the naturalistic setting chose to conduct their social behaviours when hidden from the public, thus the actual sociality event scores could be far higher. At current, however, there is no data to further investigate this.
One area to investigate further is the components of the naturalistic enclosure itself. In the wild, this gecko is found almost solely on P. rabaiensis plants, which may grow to over three metres [33]. In this study, alternative plants have been used, including wandering Jew plants, spider plants, and devil’s ivy. These plants originate from South Africa, Mexico, and Mo’orea respectively, so may not truly reflect the gecko’s native flora [33]. However, the justification for these plants is that they provide living stems and leaves for geckos to climb on and hide behind, and they increase the humidity of the enclosures. However, the full functionality of the screwpine for wild geckos is not known, and it is possible that these alternative plants do not meet all the behavioural needs of their gecko inhabitants. Further studies could be used to identify whether the screwpine has an effect on gecko behaviour.
Questionnaire responses
The responses from the questionnaire indicated that respondents showed a clear preference for naturalistic enclosures. These individuals also believed that a naturalistic enclosure provided the best welfare for its inhabitants.
Moss, et al. [14] suggest that animal visibility is critical if the attention of a visitor or audience is to be maintained. If not engaged, visitors are also less likely to interact with educational materials and are less likely to remember information about the species they encounter. For a Critically Endangered species such as L. williamsi, this conservation message is essential, especially as the pet trade has had a significant impact on numbers [33]. The construction of naturalistic settings therefore appears to be counter-intuitive. However, this study demonstrates that while a naturalistic enclosure might reduce visibility, it does enhance the public’s perception of the welfare of the animals being housed.
Reptile welfare is notoriously difficult to assess, and some scientific studies have attempted to identify measures of welfare, with varying levels of success [32]. Without facial muscles, human understanding of reptile behaviour is further hampered by their thermal range, in that cold reptiles may not display the full suite of behaviours that a reptile at its preferred optimal temperature might [42]. Members of the public are often unaware of the differences in behaviour and husbandry needs for reptiles, and may assess welfare based on assumed requirements for space and company [6]. Additionally, there are a few indicators of negative welfare that may be utilised across all reptile taxa. Few forms of stereotypy have been identified [43]. Furthermore, inactivity is sometimes cited as an indicator of impoverished welfare for some mammalian taxa [25]. However, inactivity is an important part of many reptilian activity budgets, and some behaviours, such as sun basking, may be misconstrued as inactivity [25]. To advance the use of inactivity or other behavioural indicators of welfare, activity budgets will need to be produced for animals that are experiencing good welfare, so that comparisons can be made. Physiological measures such as the validation of adrenocorticoid measurements may be required.
Though hampered by visibility, this study provides an initial investigation into the behaviour of L. williamsi in two different enclosure types that are regularly used in the captive setting, along with the visitor preference for herptile housing types. Further studies may be used to identify the behavioural indicators of gecko welfare, and the husbandry and planted environments in which they live.
There appears to be a preference among observers for naturalistic enclosures. However, zoo visitors are not always aware of what might be classed as naturalistic and true to a species’ natural habitat. The proportion of time spent hidden acts as a confounding factor for this study. Currently, there is insufficient data to suggest that turquoise blue geckos experience enhanced welfare when housed in a naturalistic setting. Overall, the findings of the survey component suggest that visitors believe naturalistic enclosures are better, yet there is insufficient data to actually identify the effects on animal inhabitants.
The authors are grateful to Mrs S Brereton and Mr R Davies for their useful comments on the manuscript. The authors would also like to thank Miss K Hunt and Mr S Nash for initiating the project, and the staff at the Animal Management Centre for supporting the research.
- Ross SR, Schapiro SJ, Hau J, Lukas KE. Space use as an indicator of enclosure appropriateness: A novel measure of captive animal welfare. Applied Animal Behaviour Science. 2009; 121(1): 42-50.
- Mallapur A, Qureshi Q, Chellam R. Enclosure design and space utilization by Indian leopards (Panthera pardus) in four zoos in Southern India. J Appl Anim Welf Sci. 2002;5(2):111-24. doi: 10.1207/S15327604JAWS0502_02. PMID: 12738580.
- Tan HM, Ong SM, Langat G, Bahaman AR, Sharma RS, Sumita S. The influence of enclosure design on diurnal activity and stereotypic behaviour in captive Malayan Sun bears (Helarctos malayanus). Res Vet Sci. 2013 Apr;94(2):228-39. doi: 10.1016/j.rvsc.2012.09.024. Epub 2012 Nov 9. PMID: 23141171.
- Brereton JE. Directions in animal enclosure use studies. Journal of Zoo and Aquarium Research. 2020a; 8(1): 1-9.
- IUCN. Summary statistics. International Union for the Conservation of Nature. 2018. http://www.iucnredlist.org/about/summary-statistics#Tables_3_4
- Melfi VA. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: a case for evidence-based zoo animal management. Zoo Biol. 2009 Nov;28(6):574-88. doi: 10.1002/zoo.20288. PMID: 19876912.
- Whittaker AL, Golder-Dewar B, Triggs JL, Sherwen SL, McLelland DJ. Identification of Animal-Based Welfare Indicators in Captive Reptiles: A Delphi Consultation Survey. Animals (Basel). 2021 Jul 5;11(7):2010. doi: 10.3390/ani11072010. PMID: 34359138; PMCID: PMC8300299.
- Andersen LL. Zoo interpretation and exhibit design: two sides of the same coin. Journal of Museum Education. 1991; 16(2): 4-6.
- Davey G. Relationships between exhibit naturalism, animal visibility and visitor interest in a Chinese Zoo. Applied Animal Behaviour Science. 2006; 96(1): 93-102.
- Fàbregas MC, Guillén-Salazar F, Garcés-Narro C. Do naturalistic enclosures provide suitable environments for zoo animals? Zoo Biol. 2012 May-Jun;31(3):362-73. doi: 10.1002/zoo.20404. Epub 2011 Jun 17. PMID: 21688309.
- Carter KC, Keane IA, Clifforde LM, Rowden LJ, Fieschi-Méric L, Michaels CJ. The Effect of Visitors on Zoo Reptile Behaviour during the COVID-19 Pandemic. Journal of Zoological and Botanical Gardens. 2021; 2(4): 664-676.
- Brereton JE. Challenges and directions in zoo and aquarium food presentation research: A review. Journal of Zoological and Botanical Gardens. 2020b; 1(1): 2.
- Kelling AS, Gaalema DE. Postoccupancy evaluations in zoological settings. Zoo Biol. 2011 Nov-Dec;30(6):597-610. doi: 10.1002/zoo.20398. Epub 2011 May 23. PMID: 21608023.
- Moss A, Esson M, Bazley S. Applied research and zoo education: The evolution and evaluation of a public talks program using unobtrusive video recording of visitor behavior. Visitor Studies. 2010; 13(1): 23-40.
- Moss A, Esson M. Visitor interest in zoo animals and the implications for collection planning and zoo education programmes. Zoo Biol. 2010 Nov-Dec;29(6):715-31. doi: 10.1002/zoo.20316. PMID: 20333734.
- Sherwen SL, Hemsworth PH. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals (Basel). 2019 Jun 17;9(6):366. doi: 10.3390/ani9060366. PMID: 31212968; PMCID: PMC6617010.
- Salas M, Laméris DW, Depoortere A, Plessers L, Verspeek J. Zoo Visitor Attitudes Are More Influenced by Animal Behaviour than Environmental Enrichment Appearance. Animals (Basel). 2021 Jun 30;11(7):1971. doi: 10.3390/ani11071971. PMID: 34209437; PMCID: PMC8300318.
- Collins C, Barr Y, McKeown S, Scheun J, Tay C, O'Riordan R. An International Investigation of the Prevalence of Negative Visitor Behaviour in the Zoo. Animals (Basel). 2023 Aug 18;13(16):2661. doi: 10.3390/ani13162661. PMID: 37627451; PMCID: PMC10451161.
- Melfi VA, McCormick W, Gibbs A. A preliminary assessment of how zoo visitors evaluate animal welfare according to enclosure style and the expression of behavior. Anthrozoös. 2004; 17(2): 98-108.
- Choo Y, Todd PA, Li D. Visitor effects on zoo orangutans in two novel, naturalistic enclosures. Applied Animal Behaviour Science. 2011; 133(1): 78-86.
- Damerell P, Howe C, Milner-Gulland EJ. Child-orientated environmental education influences adult knowledge and household behaviour. Environmental Research Letters. 2013; 8(1): 1-7.
- Woods B. Beauty and the Beast: Preferences for animals in Australia. Journal of Tourism Studies. 2000; 11(2): 25-35.
- Laina E. Regulating Sustainable Trade in Wildlife: Natural Resources and National Economies at Stake. Environmental Policy and Law. 2016; 46(6): 358.
- Durrell G, Durrell L. Breeding Mascarene wildlife in captivity. International Zoo Yearbook. 1980; 20(1); 112-119.
- Burghardt GM. Environmental enrichment and cognitive complexity in reptiles and amphibians: concepts, review, and implications for captive populations. Applied Animal Behaviour Science. 2013; 147(3): 286-298.
- Rosier RL, Langkilde T. Does environmental enrichment really matter? A case study using the eastern fence lizard, Sceloporus undulatus. Applied Animal Behaviour Science. 2011; 131(1): 71-76.
- Bashaw MJ, Gibson MD, Schowe DM, Kucher AS. Does enrichment improve reptile welfare? Leopard geckos (Eublepharis macularius) respond to five types of environmental enrichment. Applied Animal Behaviour Science. 2016; 184(1): 150-160.
- Furrer SC, Jaag K, von Stockar S, Rübel A. First experiences with free‐ranging giant day geckos (Phelsuma madagascariensis grandis, Gray 1870) in the Masoala rainforest exhibit in Zurich Zoo, Switzerland. Zoo Biology. 2006; 25(5): 409-415.
- Gusset M, Dick G. The global reach of zoos and aquariums in visitor numbers and conservation expenditures. Zoo Biol. 2011 Sep-Oct;30(5):566-9. doi: 10.1002/zoo.20369. Epub 2010 Dec 6. PMID: 21136509.
- Brereton SR, Brereton JE. Investigating Market and Conservation Education Influences on Global Zoo and Aquarium Animal Collections. Journal of Research in Social Science and Humanities. 2023a; 2(1): 25-34.
- Brereton JE, Brereton S. The effect of basking light provision on sun beetle enclosure use. Journal of Zoo and Aquarium Research. 2023b; 11(2): 283-288.
- Moszuti SA, Wilkinson A, Burman OH. Response to novelty as an indicator of reptile welfare. Applied Animal Behaviour Science. 2017; 193: 98-103.
- Flecks M, Weinsheimer F, Böhme W, Chenga J, Lötters S, Rödder D. Watching extinction happen: the dramatic population decline of the critically endangered Tanzanian Turquoise Dwarf Gecko, Lygodactylus williamsi. Salamandra. 2012; 48(1): 12-20.
- Kilawe CJ, Mchelu HA, Emily CJ. The impact of the invasive tree Cedrela odorota on the Electric Blue Gecko (Lygodactylus williamsi) and its habitat (Pandanus rabaiensis) in Kimboza Forest Reserve, Tanzania. Global Ecology and Conservation. 2022; 38: e02225.
- Stringham OC, García‐Díaz P, Toomes A, Mitchell L, Ross JV, Cassey P. Live reptile smuggling is predicted by trends in the legal exotic pet trade. Conservation Letters. 14(6); e12833.
- Meng H, Carr J, Beraducci J, Bowles P, Branch WR, Capitani C, Chenga J, Cox N, Howell K, Malonza Marchant R, Mbilinyi B, Mukama K, Msuya C, Platts PJ, Safari I, Spawls S, Shennan-Farpon Y, Burgess ND. Tanzania's reptile biodiversity: Distribution, threats and climate change vulnerability. Biological Conservation. 2016; 204(1): 72-82.
- Harrington LA, Auliya M, Eckman H, Harrington AP, Macdonald DW, D'Cruze N. Live wild animal exports to supply the exotic pet trade: A case study from Togo using publicly available social media data. Conservation Science and Practice. 2021; 3(7): e430.
- Kilawe CJ, Baltazary IS, Malila BP, Lyimo PJ, Mwakalukwa EE. Replacement of native trees by the neotropical invasive tree Cedrela odorata L. in the Kimboza Forest Reserve, Tanzania. Biological Invasions. 2023; 25(12): 3697-3710.
- Rech I, Ginal P, Rauhaus A, Ziegler T, Rödder D. Geckos in zoos: A global approach on distribution patterns of threatened geckos (Gekkota) in zoological institutions. Journal for Nature Conservation. 2023; 75: 126467.
- Martin P, Bateson P. Measuring behaviour: an introductory guide (2nd Edition). Cambridge: Cambridge University Press. 1993.
- Pandav BN, Shanbhag BA, Saidapur SK. Ethogram of courtship and mating behaviour of garden lizard, Calotes. Current Science. 2007; 93(8): 1164-1167.
- Wilkinson A, Chan HM, Hall G. Spatial learning and memory in the tortoise (Geochelone carbonaria). J Comp Psychol. 2007 Nov;121(4):412-8. doi: 10.1037/0735-7036.121.4.412. PMID: 18085925.
- Mueller-Paul J, Wilkinson A, Hall G, Huber L. Response-stereotypy in the jewelled lizard (Timon lepidus) in a radial-arm maze. Herpetology Notes. 2012; 5(2): 243.