More Information
Submitted: July 03, 2023 | Approved: July 10, 2023 | Published: July 11, 2023
How to cite this article: Mohammadian M, Bahaoddini A. IC87201, a PSD-95/nNOS Inhibitor, Ameliorates Heart Rate Variability in the Rat Model of Middle Cerebral Artery Occlusion. Insights Biol Med. 2023; 7: 001-006.
DOI: 10.29328/journal.ibm.1001024
Copyright License: © 2023 Mohammadian M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cerebral ischemia; Heart rate variability; IC87201; Middle cerebral artery occlusion; Stroke
IC87201, a PSD-95/nNOS Inhibitor, Ameliorates Heart Rate Variability in the Rat Model of Middle Cerebral Artery Occlusion
Maryam Mohammadian and Aminollah Bahaoddini*
Department of Biology, College of Sciences, Shiraz University, Shiraz, Iran
*Address for Correspondence: Aminollah Bahaoddini, Department of Biology, College of Sciences, Shiraz University, Shiraz, Iran, Email: [email protected]
Objective: Assessment of heart rate variability (HRV) is a non-invasive and reliable method to evaluate autonomic disorders after cerebral ischemia. The present study was conducted to investigate the therapeutic potential of IC87201 in reducing post-stroke cardiac dysfunction.
Materials and methods: Cerebral ischemia was induced by the middle cerebral artery occlusion (MCAO) method in 15 anesthetized adult male rats in three MCAO, MCAO+ DXM, and MCAO+ IC87201 groups, for one hour. Electrocardiogram was recorded before, and 48 hours after ischemia and drug administration, and HRV parameters were calculated from R-R intervals. In the treatment groups, IC87201 and Dextromethorphan hydrobromide monohydrate (DXM) were injected after an ischemic period.
Results: After brain ischemia, the R-R interval decreased and consequently heart rate increased. The R-R intervals were used to extract the HRV frequency and time domains, including normalized low frequency (LF), high frequency (HF), LF/HF ratio, and standard deviation of R-R interval (SDRR). Normalized LF and LF/HF ratio enhanced 48 hours after ischemia, while normalized HF and SDRR significantly reduced compared to the pre-ischemic state. All HRV parameters had returned to their pre-ischemic level 48 hours after IC87201 and DXM administration, except SDRR, which recovered only in the IC87201 administered group.
Conclusion: Based on our findings, it can be concluded that cerebral ischemia significantly worsens HRV parameters as a result of sympathetic overactivity. These changes were reversed by administering DXM and IC87201, but IC87201 has generally been more effective in lowering lesions. As a result, IC87201 can be introduced as an effective substance for the treatment of post-ischemic cardiac side effects.
Stroke is the second reason for death and the third leading cause of disability in the world [1]. The reason for stroke can be a rupture of a blood vessel inside the brain (hemorrhagic stroke) or occlusion of a cerebral artery (ischemic stroke) [2], the latter with a prevalence of 87% is the most common cause [3]. There is considerable evidence that cerebral ischemia has deleterious effects on cardiac function, and cardiac-related death is one of the leading causes of death post-stroke [4]. The brain-heart axis is a useful model for comprehending how the autonomic central nervous system, which can be damaged after a stroke, interacts with the heart’s autonomic regulation [5,6].
The heart rate variability (HRV) analysis is a noninvasive technique for examining autonomic function, that is required just an electrocardiogram (ECG) recording. HRV allows for the analysis of parameters related to parasympathetic or sympathetic activity, as well as their variations under various conditions, in the time and frequency domains [7]. HRV is defined as the fluctuations in the intervals between normal heartbeats and can be extracted from R-R interval variation in an electrocardiogram [8]. HRV is constantly altering as a result of the dynamic nature of autonomic nervous system (ANS) activity. A single HRV measurement represents the immediate activity of the ANS at a particular time. Reduced HRV is a sign of diminished ANS responses, and the severity of the disease is related to this process. On the other hand, a rise in HRV would signify the return of ANS modulation [9]. It appears that the autonomic dysfunction continues during the chronic phase of stroke. In fact, ischemic stroke usually increases and decreases sympathetic and parasympathetic markers, respectively [10], which may cause cardiac autonomic disorder [11,12], myocardial damage [13-15], and changes in heart rate variability [16]. So, there is rising attention to HRV analysis as a potential tool for prognosis and comprehension of underlying mechanisms in post-stroke autonomic dysfunction.
Improved cardiac function may be achieved with the aid of substances that have the therapeutic potential to treat stroke-induced brain injury. Several studies have examined the use of NMDA receptor antagonists and preventing excitotoxicity as one of the most significant damage-causing mechanisms in cerebral ischemia due to numerous side effects such as nausea, vomiting, cardiovascular and psychomimetic, however, they have failed to become a therapeutic strategy for ischemic stroke [17-20]. Therefore, targeting NMDA receptor downstream signaling pathways can be considered as an alternative strategy. Small molecules have been developed recently that appear to be effective in reducing post-stroke injuries. IC87201, which Florio identified in 2009, is one of those chemical agents [21].
IC87201 affects the proteins attached to the intracellular surface of NMDA receptors and inhibits the interaction between postsynaptic density protein 95 (PSD-95) and n-NOS proteins that reduce nitrogenic stress and excitotoxicity injuries without preventing NMDA receptor postsynaptic currents [22,23]. Therefore, for the first time, we designed this research to investigate the therapeutic potential of IC87201 on HRV alteration induced by cerebral ischemia. Dextromethorphan hydrobromide monohydrate (DXM), an NMDA receptor antagonist, has additionally been used to compare the effects of blocking the NMDA receptor by controlling its activity through intracellular signaling pathways.
Animals and drugs
Animal procedures were performed in accordance with the local Ethics Committee of Shiraz University, Shiraz, Iran. The research work was approved by the Ethics Committee of the Biology Department, Shiraz University (SU-9531466). Adults Sprague- Dawley male rats, weighing 275–350 g obtained from the Comparative and Experimental Medical Center of Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. The rats were housed in an animal house with a controlled light cycle and temperature and available water and food access. Rats were acclimatized for one week prior to surgery. IC87201 (CAS number: 866927-10-8) and Dextromethorphan hydrobromide monohydrate (CAS number: 6700-34-1) were purchased from Tocris Bioscience (United Kingdom) and Sigma Aldrich companies, respectively.
Middle cerebral artery occlusion procedure
Transient brain ischemia was induced using the intraluminal filament technique described by Koizumi. Animals were anesthetized with intraperitoneal (i.p.) administration of Ketamine (100 mg/Kg) and Xylazine (10 mg/Kg). The right common carotid artery (CCA) was exposed through a midline incision on the neck and then carefully dissected from the vagus nerve and its sheath, external carotid artery (ECA), and internal carotid artery (ICA). A heat-rounded head and silicon coated 4–0 monofilament nylon suture was inserted into the common carotid artery and advanced into the lumen of the ICA until it blocked the origin of the middle cerebral artery (MCA). One hour after MCAO, reperfusion was performed by withdrawing the suture. Finally, the incision was sutured and sprayed with oxytetracycline. The core body temperature was maintained at 37 °C ± 0.8 °C using a rectal thermometer and a heating pad throughout and after the surgery [24].
Experimental protocols
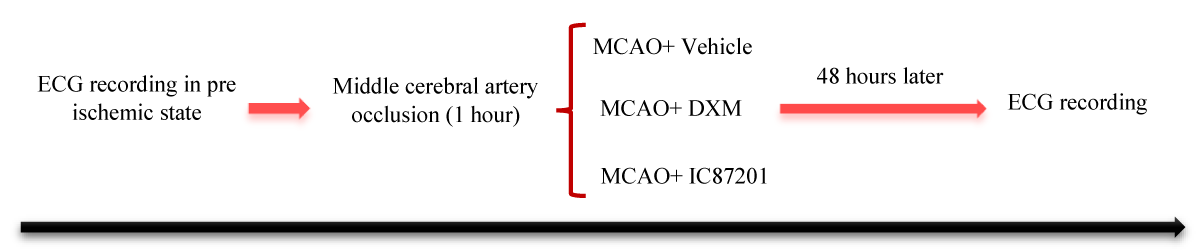
The experimental protocol is shown in Figure 1. The rats (n = 15) were randomly assigned into three groups by block randomization: MCAO, MCAO+ DXM, and MCAO+ IC87201. They were anesthetized with intraperitoneally (i.p.) administration of Ketamine (100 mg/kg) and Xylazine (10 mg/kg) [25], and core temperature was maintained at 37 °C using a rectal thermometer and a heating pad. Then, the electrocardiogram (ECG) recording leads were inserted in the prone position. In all groups, The ECG was monitored using the lead II configuration. The ECG signal was continuously displayed on a computer connected to a PowerLab system (AD Instrument Company, Australia) before, and 48 hours after cerebral ischemia induction. After the ischemic period ends, vehicle, IC87201 (10 mg/kg) [26], and Dextromethorphan hydrobromide monohydrate (DXM) (50 mg/kg) [27] were injected intraperitoneally in the MCAO, MCAO+ DXM, and MCAO+ IC87201 groups, respectively. And the ECG was recorded separately in the animals of each group. The frequency domains of HRV including low-frequency (LF, 0.04 Hz – 0.15 Hz), and high-frequency (HF, 0.15 Hz – 0.4 Hz) bands were calculated from RR intervals. Then normalized power in the LF and HF bands was calculated as LF (n.u.) = LF/ (LF + HF), HF (n.u.) = HF/ (LF + HF), and from the two indices the LF/HF ratio was calculated. Also. The standard deviation of R-R interval (SDRR) as a presentation of total HRV was evaluated [28]. In all three groups, before surgery, a baseline electrocardiogram was recorded, as the sham group (negative control), which is mentioned in the study as the pre-ischemic state.
Figure 1: Experimental procedure.
Neurological deficits evaluation
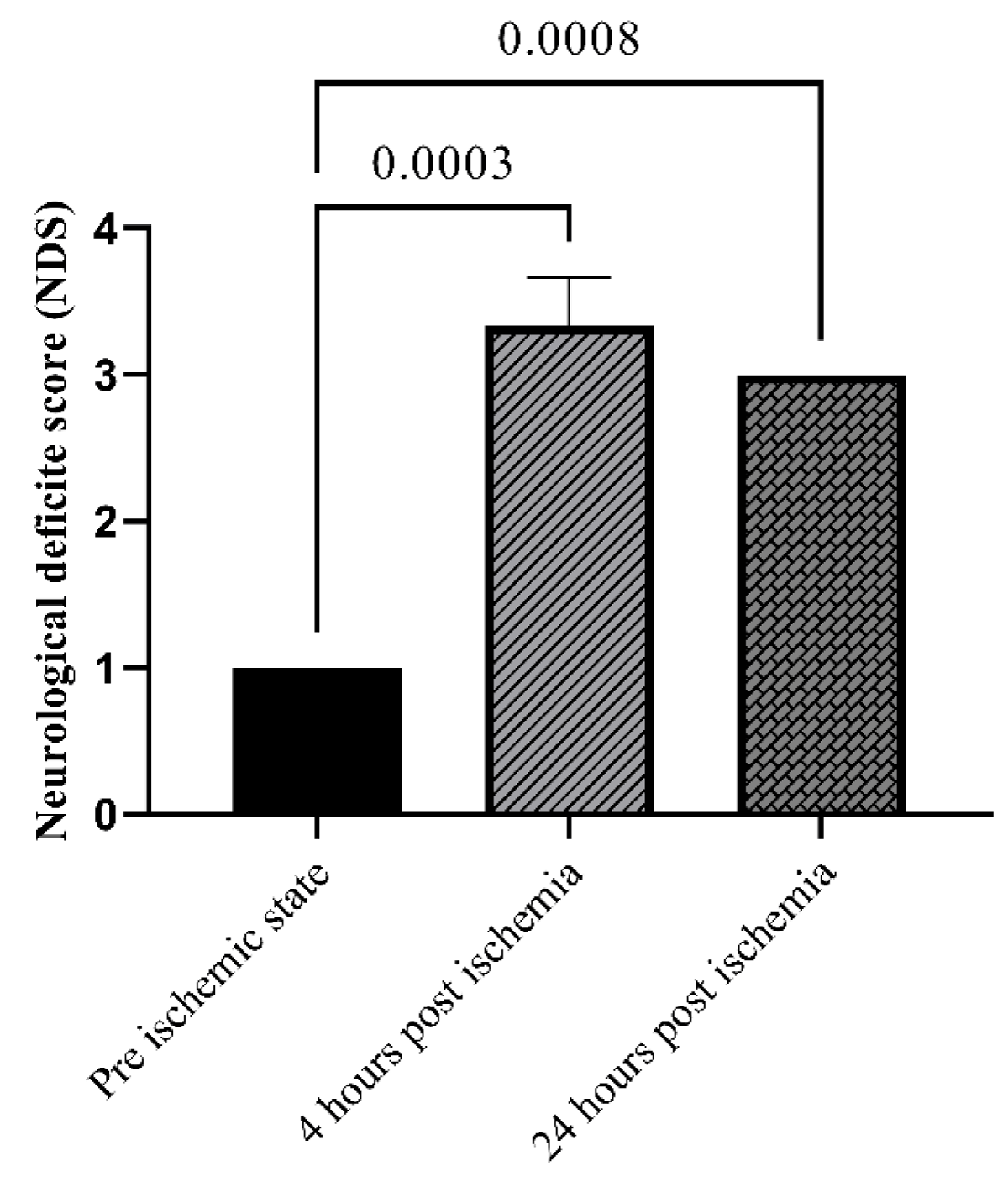
Neurological deficit scores (NDS) in both groups were performed as follows: pre-stroke, 4- and 24-hours post-stroke. Neurological findings were scored on a 5-point scale. The criterion of the evolution was as follows: grade 1 had no observable neurological deficit, grade 2 was given to rats that showed flexion of contra lateral torso or forelimb upon lifting by their tail, or failure to extend their forepaw when suspended vertically, forelimb flexion and shoulder adduction, grade 3 was for circling to the contralateral side of the middle cerebral artery occluded hemisphere when the animal is held by the tail on a flat surface, but with normal posture at rest, grade 4 was assigned to loss of righting reflex and decreased resistance to lateral push, and finally, grade 5 was for no spontaneous motor activity [29]. If the animal score was 1 or died from brain ischemia, they were excluded from the study and were replaced with another one.
Statistical analysis
All data were analyzed by Graph Pad Prism software (version 9. 1. 0). And expressed as mean ± SEM. Statistical differences were measured by one-way analysis of variance (ANOVA) combined with the LSD post hoc test for multiple group comparisons. The behavioral scores for Garcia neurological test were analyzed using repeated-measures ANOVA. Values of p < 0.05 were considered significant.
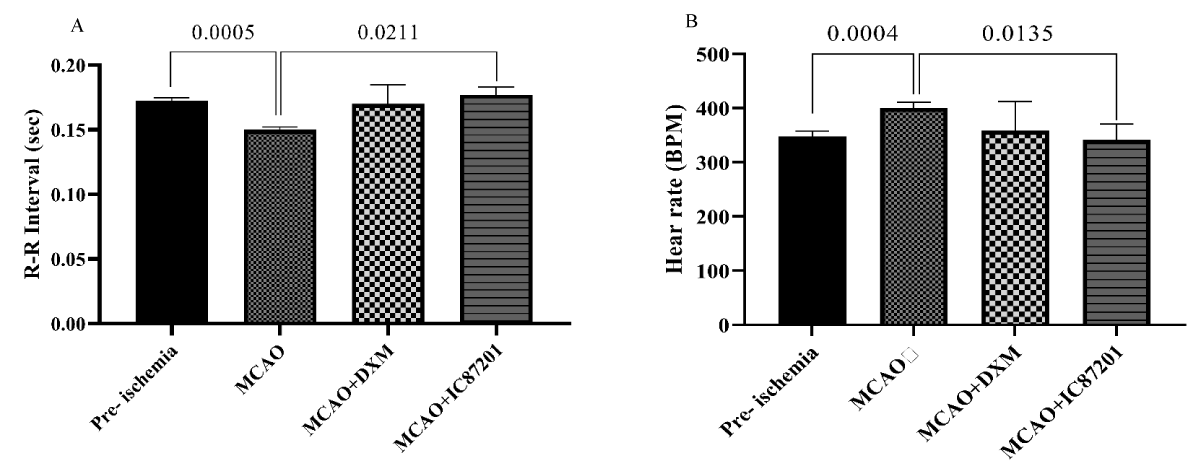
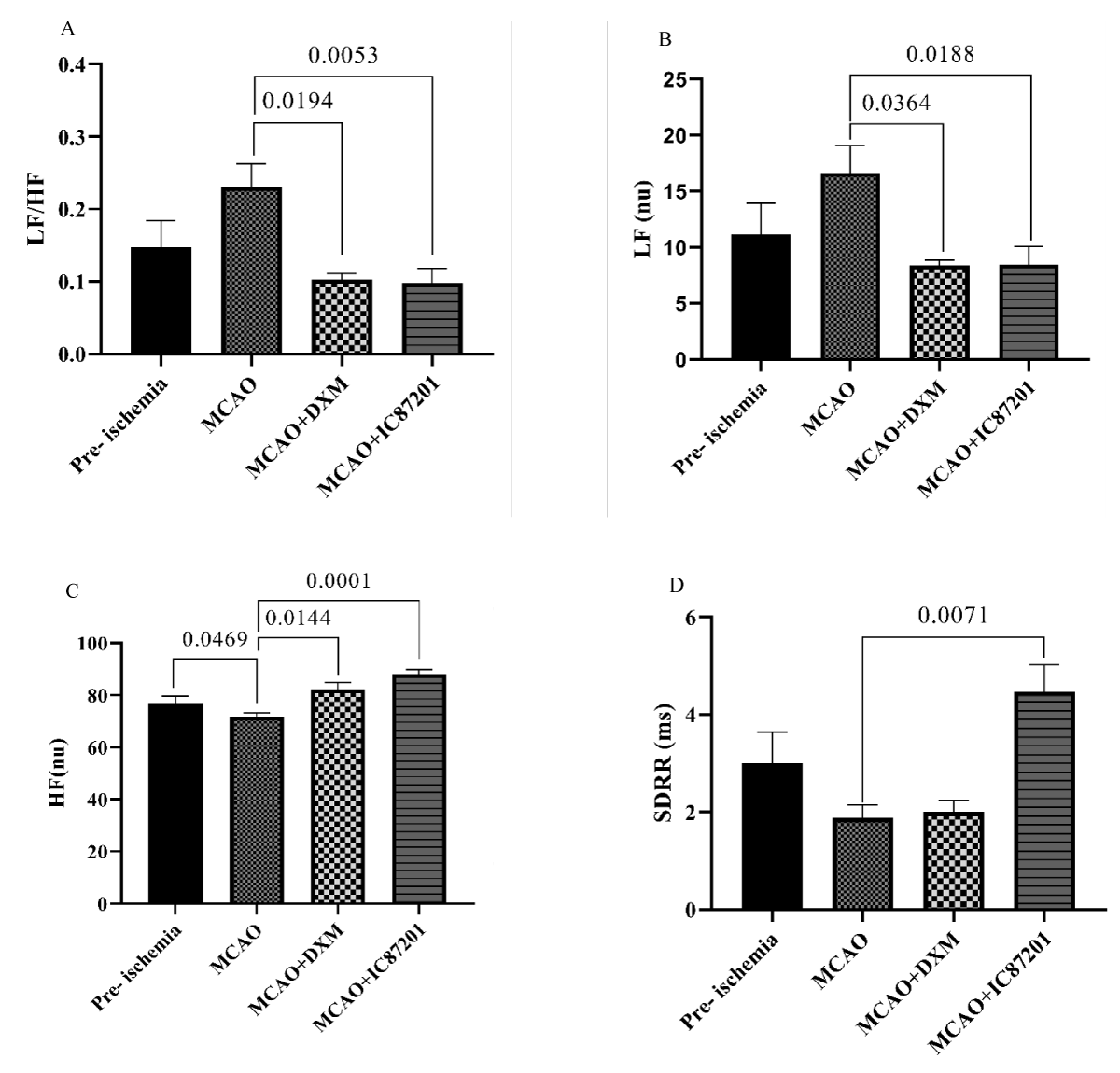
All animals were evaluated for their neurological deficit pre-stroke, 4- and 24-hours post-stroke. As shown in Figure 2, NDS as an indicator of the ischemia induction, is significantly higher post-ischemia (p < 0.05). 48 hours after ischemia, the R-R interval in the MCAO group was noticeably shorter than it was in the pre-ischemic state. In the IC87201 administered group, the RR interval significantly returned to normal levels, whereas this recovery was not seen in the DXM group (Figure 3A). Additionally, heart rate (HR) in the ischemic state was markedly elevated and returned to normal in IC87201 administered group but not the DXM group (Figure 3B). Figure 4A displays the LF/HF ratio value among animals subjected to stroke and drug administration. 48 hours following brain ischemia, this ratio increased in the MCAO group, but this rise was not statistically significant. The LF/HF ratio returned to the pre-ischemic state in the presence of IC87201 and DXM (Figure 4A). Additionally, normalized LF and HF were evaluated separately, and it was discovered that following ischemia, LF (n.u.) increased and HF (n.u.) decreased compared to the pre-ischemic state, although LF (n.u.) elevation was not statistically significant. Figure 4, parts B and C, it`s illustrated that both of these factors returned to their pre-ischemic states in the IC87201 and DXM receiving groups. The only time domain parameter assessed in this study, the standard deviation of R-R intervals (SDRR), decreased following cerebral ischemia and increased in the presence of IC87201 but not DXM to the normal level (Figure 4D).
Figure 2: Evaluation of neurobehavioral function using Neurological deficit score (NDS) pre, 4 and 24 hours post ischemia. Data are mean ± SEM.
Figure 3: R-R interval (A) and heart rate (B) recording pre- ischemia, and 48 hours post- ischemia in MCAO, MCAO + DXM, and MCAO + IC87201 groups. Data are mean ± SEM.
Figure 4: LF/HF ratio (A), normalized LF (B), normalized HF (C), and SDRR (D) recording pre- ischemia, and 48 hours post- ischemia in MCAO, MCAO + DXM, and MCAO + IC87201 groups. Data are mean ± SEM.
Stroke is one of the fatal diseases that have a significant impact in a variety of situations. The acute and chronic phases of stroke frequently involve autonomic dysfunction. HRV analysis as a noninvasive method has garnered a lot of interest in clinical testing to evaluate autonomic function. HRV frequency domain measurements approximate the distribution of absolute or relative power into frequency bands. The variability in measurements of the inter-beat interval (IBI), or the interval between heartbeats, is measured by time-domain indices of HRV [30]. In the present study, R-R intervals were recorded and HRV frequency and time domains were extracted from it. The LF (n.u.) and HF (n.u.) were measured as indicators of sympathetic and parasympathetic activity, respectively. Further evidence of sympathovagal balance was provided by the LF/HF ratio. As a representation of total HRV, the SDRR factor was also calculated.
Sympathetic predominance during an ischemic stroke along with the catecholamine surge, results in cardiac autonomic dysfunction [10,11]. Patients with ischemic stroke exhibit impaired autonomic nervous system activity in the form of persistent sympathetic/parasympathetic tonus imbalance and decreased HRV [31]. According to our observation in the MCAO group, increased LF, decreased HF, and sympathovagal imbalance toward sympathetic overactivation, which is characterized by an increase in the LF/HF ratio, all point to impaired autonomic function.
Several studies have looked into the post-stroke autonomic nervous system’s dysfunction. Stroke patients, especially those with right-sided ischemia, show lower total HRV and HF and elevated LF and LF/HF ratio which denotes an increase in sympathetic system activity [5,32,33]. The standard deviation of R-R intervals (SDRR) also known as the standard deviation of normal to normal intervals or (SDNN) [34] is considered a representation of total HRV. In fact, the SDRR is the standard deviation of the intra-beats interval for all sinus beats. Both of these parameters measure how these intervals vary over time [30]. It has been shown that HRV time domain measurements, including SDNN, were lower in acute-stage stroke patients compared to healthy control. Several studies have suggested that cerebral asymmetries may play a role in automatic cardiac control. In other words, the sympathetic and parasympathetic responses are primarily mediated by the right and left insular cortex, respectively [35]. Furthermore, when right and left-sided insular strokes were compared, it was found that right-sided middle cerebral artery strokes had significantly lower SDNN values [15,36]. In light of this, patients with right-sided strokes tended to have increased sympathetic cardiac modulation, which was supported by a change in the sympathovagal balance in favor of increased sympathetic activity [11,37]. Predominant sympathetic overactivation and reduction of heart rate variability and changes in its related parameters as a result of inducing right hemisphere ischemia in this research are consistent with previously mentioned studies.
Our findings revealed that all of the aforementioned HRV parameters recovered to their pre-ischemic states in the groups receiving DXM and IC87201, with the exception of SDRR, which was only affected by IC87201. Both of these substances interfere with NMDA receptors. Since one of the main contributors to cerebral ischemia-induced injury is excitotoxicity via NMDA receptors, targeting these receptors can be a suitable therapeutic approach for preventing brain damage and subsequent cardiac dysfunction. The neuroprotective effects of DXM, as a non-competitive NMDA receptor antagonist, have been investigated and confirmed in numerous post-cerebral ischemia studies [38]. However, the use of NMDA receptor antagonists cannot be considered an appropriate treatment solution due to numerous side effects [1]. Recently, targeting intracellular signaling pathways of these receptors has been considered as an alternative strategy to lessen the severity of ischemia-induced damage.
IC87201 as a de novo small molecule can disrupt protein-protein interaction between PSD-95 and n-NOS in the intracellular part of the NMDA receptor without effect on its postsynaptic currents. Therefore, it reduces the production of nitric oxide without affecting the catalytic activity of the n-NOS and, as a result, prevents damage caused by nitrogen free radicals. These features introduce IC87201 as a beneficial candidate to reduce cerebral ischemia injuries and subsequent cardiac complications.
In the present study, both DXM and IC87201 successfully reduced cardiac damage induced by cerebral ischemia, which may be attributed to the neuroprotective properties of these agents. In regional middle cerebral artery stroke, insular infarction is the primary sign which affects cardiovascular autonomic function [39]. Considering the expression of NMDA receptors in the insular cortex and its key role in controlling the ANS activity, the resulting cardiac effect of DXM and IC87201 can be attributed to their central improving function on the insula. Some researchers have reported that insular cortex NMDA receptor activation leads to sympathetic hyperactivity [40] which is consistent with our findings. By contrasting the effects of IC87201 and DXM, it is clear that modulating the activity of the NMDA receptor, either by blocking it entirely or by targeting its downstream pathways, has a significant positive impact on heart rate variability. IC87201, however, has been more successful in improving some parameters. Therefore, its potential as an effective substance can therefore be examined more precisely.
In general, our results support previous studies on the brain-heart axis role during acute ischemic stroke. The right middle cerebral artery occlusion results in sympathetic overactivation and decreased HRV. In the present study, for the first time, the impact of IC87201 on reducing post-stroke cardiac dysfunction was examined, and it was discovered that IC87201 can significantly recover these injuries. Therefore, a more comprehensive investigation of IC87201`s therapeutic potential is required.
We thank Miss Hajar Ebrahimiyan for their helpful discussions and comments.
Grant: This study was supported financially by Ph.D. grant no. SU9531466 from Shiraz University.
Author contributions: M.M. conducted animal surgery, performed behavioral tests, analyzed the results, and wrote the manuscript draft. A.B. designed the study, supervised the experiments, and edited the manuscript. All authors approved the final version to be published.
- Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 2018 Mar 13;11(1):15. doi: 10.1186/s13041-018-0357-8. PMID: 29534733; PMCID: PMC5851248.
- Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020 Apr 1;1866(4):165260. doi: 10.1016/j.bbadis.2018.09.012. Epub 2018 Sep 15. PMID: 31699365; PMCID: PMC6981280.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008 Nov;26(4):871-95, vii. doi: 10.1016/j.ncl.2008.07.003. PMID: 19026895.
- Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S; VISTA Investigators. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007 Aug;38(8):2295-302. doi: 10.1161/STROKEAHA.106.471813. Epub 2007 Jun 14. PMID: 17569877.
- Chen CF, Lin HF, Lin RT, Yang YH, Lai CL. Relationship between ischemic stroke location and autonomic cardiac function. J Clin Neurosci. 2013 Mar;20(3):406-9. doi: 10.1016/j.jocn.2012.02.047. Epub 2012 Dec 7. PMID: 23219823.
- Manea MM, Comsa M, Minca A, Dragos D, Popa C. Brain-heart axis--Review Article. J Med Life. 2015 Jul-Sep;8(3):266-71. PMID: 26351525; PMCID: PMC4556904.
- Buitrago-Ricaurte N, Cintra F, Silva GS. Heart rate variability as an autonomic biomarker in ischemic stroke. Arq Neuropsiquiatr. 2020 Nov;78(11):724-732. doi: 10.1590/0004-282X20200087. PMID: 33331466.
- Singh N, Moneghetti KJ, Christle JW, Hadley D, Froelicher V, Plews D. Heart Rate Variability: An Old Metric with New Meaning in the Era of Using mHealth technologies for Health and Exercise Training Guidance. Part Two: Prognosis and Training. Arrhythm Electrophysiol Rev. 2018 Dec;7(4):247-255. doi: 10.15420/aer.2018.30.2. PMID: 30588312; PMCID: PMC6304793.
- Guan L, Collet JP, Mazowita G, Claydon VE. Autonomic Nervous System and Stress to Predict Secondary Ischemic Events after Transient Ischemic Attack or Minor Stroke: Possible Implications of Heart Rate Variability. Front Neurol. 2018 Mar 5;9:90. doi: 10.3389/fneur.2018.00090. PMID: 29556209; PMCID: PMC5844932.
- Sörös P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012 Feb;11(2):179-88. doi: 10.1016/S1474-4422(11)70291-5. PMID: 22265213.
- Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004 Sep;35(9):2094-8. doi: 10.1161/01.STR.0000138452.81003.4c. Epub 2004 Jul 22. PMID: 15272134.
- Colivicchi F, Bassi A, Santini M, Caltagirone C. Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke. 2005 Aug;36(8):1710-5. doi: 10.1161/01.STR.0000173400.19346.bd. Epub 2005 Jul 14. PMID: 16020766.
- Fyfe-Johnson AL, Muller CJ, Alonso A, Folsom AR, Gottesman RF, Rosamond WD, Whitsel EA, Agarwal SK, MacLehose RF. Heart Rate Variability and Incident Stroke: The Atherosclerosis Risk in Communities Study. Stroke. 2016 Jun;47(6):1452-8. doi: 10.1161/STROKEAHA.116.012662. Epub 2016 May 5. PMID: 27217501; PMCID: PMC4880420.
- Huikuri HV, Stein PK. Heart rate variability in risk stratification of cardiac patients. Prog Cardiovasc Dis. 2013 Sep-Oct;56(2):153-9. doi: 10.1016/j.pcad.2013.07.003. Epub 2013 Aug 12. PMID: 24215747.
- Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke. 2015 Aug;10(6):796-800. doi: 10.1111/ijs.12573. PMID: 26202709.
- Bodapati RK, Kizer JR, Kop WJ, Kamel H, Stein PK. Addition of 24-Hour Heart Rate Variability Parameters to the Cardiovascular Health Study Stroke Risk Score and Prediction of Incident Stroke: The Cardiovascular Health Study. J Am Heart Assoc. 2017 Jul 21;6(7):e004305. doi: 10.1161/JAHA.116.004305. PMID: 28733431; PMCID: PMC5586256.
- Albers GW, Atkinson RP, Kelley RE, Rosenbaum DM. Safety, tolerability, and pharmacokinetics of the N-methyl-D-aspartate antagonist dextrorphan in patients with acute stroke. Dextrorphan Study Group. Stroke. 1995 Feb;26(2):254-8. doi: 10.1161/01.str.26.2.254. PMID: 7831698.
- Diener HC, AlKhedr A, Busse O, Hacke W, Zingmark PH, Jonsson N, Basun H; Study group. Treatment of acute ischaemic stroke with the low-affinity, use-dependent NMDA antagonist AR-R15896AR. A safety and tolerability study. J Neurol. 2002 May;249(5):561-8. doi: 10.1007/s004150200065. PMID: 12021946.
- Grotta J, Clark W, Coull B, Pettigrew LC, Mackay B, Goldstein LB, Meissner I, Murphy D, LaRue L. Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke. 1995 Apr;26(4):602-5. doi: 10.1161/01.str.26.4.602. PMID: 7709405.
- Wood PL, Hawkinson JE. N-methyl-D-aspartate antagonists for stroke and head trauma. Expert Opin Investig Drugs. 1997 Apr;6(4):389-97. doi: 10.1517/13543784.6.4.389. PMID: 15989606.
- Florio SK, Loh C, Huang SM, Iwamaye AE, Kitto KF, Fowler KW, Treiberg JA, Hayflick JS, Walker JM, Fairbanks CA, Lai Y. Disruption of nNOS-PSD95 protein-protein interaction inhibits acute thermal hyperalgesia and chronic mechanical allodynia in rodents. Br J Pharmacol. 2009 Sep;158(2):494-506. doi: 10.1111/j.1476-5381.2009.00300.x. PMID: 19732061; PMCID: PMC2757689.
- Lee WH, Xu Z, Ashpole NM, Hudmon A, Kulkarni PM, Thakur GA, Lai YY, Hohmann AG. Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacology. 2015 Oct;97:464-75. doi: 10.1016/j.neuropharm.2015.05.038. Epub 2015 Jun 10. PMID: 26071110; PMCID: PMC4539046.
- Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, Lu W, Ji X, Zhou QG, Zhu DY. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010 Dec;16(12):1439-43. doi: 10.1038/nm.2245. Epub 2010 Nov 21. Erratum in: Nat Med. 2011 Sep;17(9):1153. PMID: 21102461.
- Owjfard M, Bigdeli MR, Safari A, Namavar MR. Effects of nicorandil on neurobehavioral function, BBB integrity, edema and stereological parameters of the brain in the sub-acute phase of stroke in a rat model. J Biosci. 2020;45:49. PMID: 32345775.
- Naderi Y, Sabetkasaei M, Parvardeh S, Moini Zanjani T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol. 2017 Apr;28(2 and 3-Spec Issue):214-222. doi: 10.1097/FBP.0000000000000297. PMID: 28257293.
- Smith AE, Xu Z, Lai YY, Kulkarni PM, Thakur GA, Hohmann AG, Crystal JD. Source memory in rats is impaired by an NMDA receptor antagonist but not by PSD95-nNOS protein-protein interaction inhibitors. Behav Brain Res. 2016 May 15;305:23-9. doi: 10.1016/j.bbr.2016.02.021. Epub 2016 Feb 22. PMID: 26909849; PMCID: PMC4808404.
- Bokesch PM, Marchand JE, Connelly CS, Wurm WH, Kream RM. Dextromethorphan inhibits ischemia-induced c-fos expression and delayed neuronal death in hippocampal neurons. Anesthesiology. 1994 Aug;81(2):470-7. doi: 10.1097/00000542-199408000-00026. PMID: 8053597.
- Verma AK, Aarotale PN, Dehkordi P, Lou JS, Tavakolian K. Relationship between Ischemic Stroke and Pulse Rate Variability as a Surrogate of Heart Rate Variability. Brain Sci. 2019 Jul 10;9(7):162. doi: 10.3390/brainsci9070162. PMID: 31295816; PMCID: PMC6680838.
- Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004 Feb 9;15(2):357-61. doi: 10.1097/00001756-200402090-00029. PMID: 15076768.
- Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017 Sep 28;5:258. doi: 10.3389/fpubh.2017.00258. PMID: 29034226; PMCID: PMC5624990.
- Oka H. Heart rate variability and neurological disorders. Clinical assessment of the autonomic nervous system. 2017; 179-197.
- Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, Ayata C, Zhu M, Schwamm LH, Sorensen AG. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006 May 9;66(9):1325-9. doi: 10.1212/01.wnl.0000206077.13705.6d. Epub 2006 Mar 8. PMID: 16525122.
- Dütsch M, Burger M, Dörfler C, Schwab S, Hilz MJ. Cardiovascular autonomic function in poststroke patients. Neurology. 2007 Dec 11;69(24):2249-55. doi: 10.1212/01.wnl.0000286946.06639.a7. PMID: 18071145.
- Chevalier G, Sinatra ST. Emotional stress, heart rate variability, grounding, and improved autonomic tone: clinical applications. Integrative Medicine. 2011; 10(3): 16-21.
- Marins FR, Iddings JA, Fontes MA, Filosa JA. Evidence that remodeling of insular cortex neurovascular unit contributes to hypertension-related sympathoexcitation. Physiol Rep. 2017 Mar;5(5):e13156. doi: 10.14814/phy2.13156. PMID: 28270592; PMCID: PMC5350170.
- Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, Zingler C, Benecke R. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol. 2013 Jan;20(1):153-9. doi: 10.1111/j.1468-1331.2012.03818.x. Epub 2012 Jul 27. PMID: 22834894.
- Micieli G, Cavallini A. The autonomic nervous system and ischemic stroke: a reciprocal interdependence. Clin Auton Res. 2008 Dec;18(6):308-17. doi: 10.1007/s10286-008-0495-7. Epub 2008 Oct 11. PMID: 18850312.
- Werling LL, Lauterbach EC, Calef U. Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist. 2007 Sep;13(5):272-93. doi: 10.1097/NRL.0b013e3180f60bd8. PMID: 17848867.
- Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, Caplan LR. Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Arch Neurol. 2005 Jul;62(7):1081-5. doi: 10.1001/archneur.62.7.1081. PMID: 16009763.
- Marins FR, Limborço-Filho M, Iddings JA, Xavier CH, Biancardi VC, Stern JE, Ramiro Diaz J, Oppenheimer SM, Filosa JA, Peliky Fontes MA. Tachycardia evoked from insular stroke in rats is dependent on glutamatergic neurotransmission in the dorsomedial hypothalamus. Eur J Neurol. 2021 Nov;28(11):3640-3649. doi: 10.1111/ene.14987. Epub 2021 Jul 9. PMID: 34152065.