More Information
Submitted: April 18, 2022 | Approved: May 05, 2022 | Published: May 06, 2022
How to cite this article: Bereda G. Anthelmintic agents: vermicide and vermifuge. Insights Biol Med. 2022; 6: 001-008.
DOI: 10.29328/journal.ibm.1001020
Copyright License: © 2022 Bereda G. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations: Anthelmintic; Vermicide; Vermifuge
Keywords: ADRS: Adverse Drug Reactions; ATP: Adenosine Triphosphate; CYP450: Cytochrome P450; DI: Drug Interaction; GABA: Gamma-Amino Butyric Acid; PFOR: Pyruvate Ferredoxin Oxidoreductase; PH: Potenz Hydrogen; nAChRs: Nicotinic Acetylcholine Receptors; P-gp: Pglycoprotein
Anthelmintic agents: vermicide and vermifuge
Gudisa Bereda1*
Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia
*Address for Correspondence: Gudisa Bereda, Department of Pharmacy, Negelle Health Science College, Guji, Ethiopia; Email: [email protected]
Helminthiasis is also known as worm infection, is any macroparastic disease of humans and other animals in which a part of the body is infected with parasitic worms known as helminths. Anthelmintic agents are medicines that used for treatment and inhibition of parasitic infections caused by helminths; which involve both flat worms, such as, flukes and tapeworms and round worms, such as, nematodes. Anthelmintics are categorized into groups depending on the basis of their identical chemical structure and mode of action. Thiabendazole, mebendazole, and albendazole belong to benzimidazoles group of antihelmenthic medicines. From benzimidazoles group of antihelmenthic, thiabendazole was first discovered in 1961 and already a mentioned number of more benzamidazoles were interpolated as wide spectrum anthelmintics. Praziquantel has a particular effect on the enveloping layer of trematodes and increases permeability of calcium ion influx leading to uncontrolled muscle contraction and paralysis. Praziquantel has a particular toxic effect on schistosome parasites, where its mode of action has been resulted more extensively than in cestodes. Coadministration of mebendazole with CYP450 inhibitors medications such as cimetidine, ketoconazole and etc may be increases plasma levels of mebendazole, by extending the half-life and decreasing plasma clearance.
The helminths are invertebrates described by lengthen its flat or round bodies. In medical oriented plot the flatworms or platyhelminths (platy comes from Greek which means “flat”) involve flukes and tapeworms [1]. Roundworms are nematodes (nemato comes from the Greek which means “thread”). These classes of helminths are subdivided according to the host organ in which they reside, example, lung flukes, extraintestinal tapeworms, and intestinal roundworms. Helminth infections are the only most present diseases in advancing and advanced countries [2,3]. World Health Organization evaluates that an astonishingly 2 billion people harbour parasitic worm infections and also parasitic worms infect livestock and crops, affecting food production with resulted in economic impacts, and have significant impact on the infection of domestic pets. Assuredly, the escort animal market is a considerable economic deliberation for animal health companies undertaking medicine discovery programmes [4,5].
There are three types of Helminths which are: 1) Nematodes (round worms) involves ascarids (ascaris), filarias, hookworms, pinworms (enterobius), and whipworms (trichuristrichiura), and 2) Cestodes (tape worms) involves multiple species of flat worms, taenia saginatum, taenia solium (cysticercosis, hydatid (echinococcus), and 3) Trematodes (flukes) involves liver flukes, lung flukes, schistosoma [6,7] (Figure 1).
Figure 1: Pictures of type of Helminthes.
Anthelmintic agents
Anthelmintic agents are medicines that are used for treatment of infections with parasitic worms. This involves both flat worms, such as, flukes and tapeworms and round worms, such as, nematodes. They have great significance for human tropical medication and for veterinary medicine [8,9]. Anthelmintic drugs are act through two mechanisms: (1) Vermicide (kill) is used to kill parasitic intestinal worms [10]. (2) Vermifuge (eject) is used to destroy or eject worms in the intestine [11]. Albendazole and mebendazole have been selected as concentrated medicine administration programs and have best activity against ascariasis and hookworm infections [12]. Anthelmintic drugs are divided into groups depending on the basis of their identical chemical structure and mode of action [13]. There are only a few pivotal groups of anthelminthic drugs which are briefly discussed in turn below.
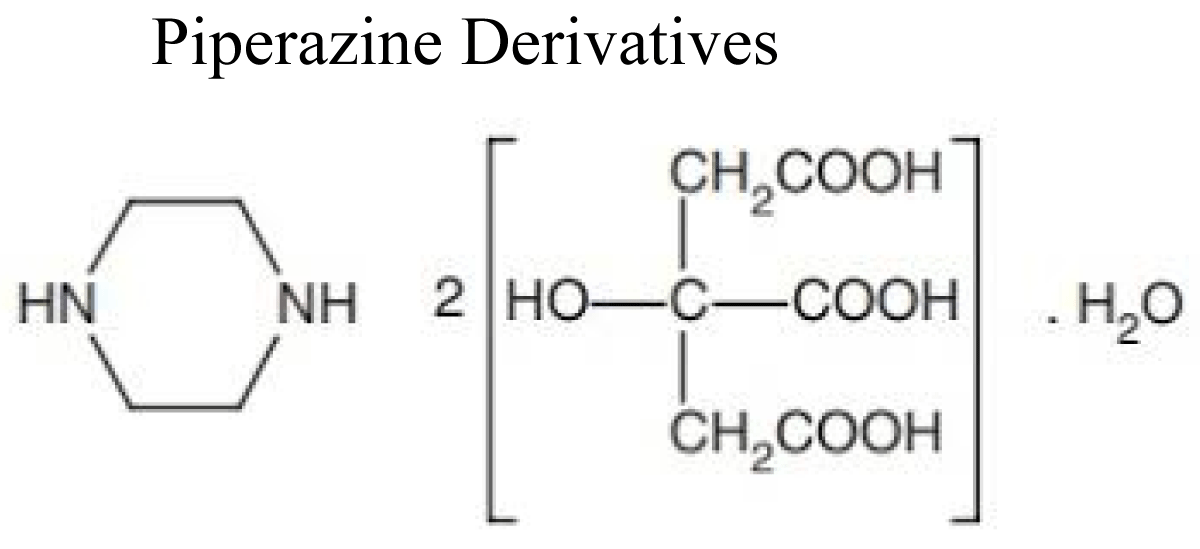
Piperazine derivatives (piperazine citrate, diethyl-carbamazine).
Piperazine is an organic compound that consist a six-membered ring containing two nitrogen atoms at opposite positions in the ring [14] (Figure 2).
Figure 2: Chemical structure of Piperazine.
Mechanism of action: Piperazine acts as a weak GABA (4-aminobutyric acid)-mimetic in ascaris summation and causes a flaccid, and reversible paralysis of body wall muscles of helminths. Single-channel recordings of piperazine display a least efficacy and partial agonist at GABA-gated chloride channels and piperazine causes muscle paralysis of the round worm such ascaris lumbricoides as by suppressing the consequences of Ach at the neuromuscular junction [15,16].
Use: Piperazine used for treatment or eradication of roundworms (ascariasis) and pinworm (enterobiasis, oxyuriasis) [17].
Adverse drug reactions: The side effects of piperazine includes bronchospasm; nystagmus; abdominal discomfort; rashes; SJS; angioedema; ataxia, muscular blockage, angioedema, myoclonic contractions, dizziness, paraesthesia [18].
Contraindications: Piperazine is not given to individuals with epilepsy; severe kidney impairment, pregnant women [19].
Drug interactions: If high dose of piperazine and pyrantel coadministered; they have antagonistic effects. If piperazine is administered coincidentally with phenothiazines medications; piperazine potentiates extrapyramidal effects of phenothiazines medications [20].
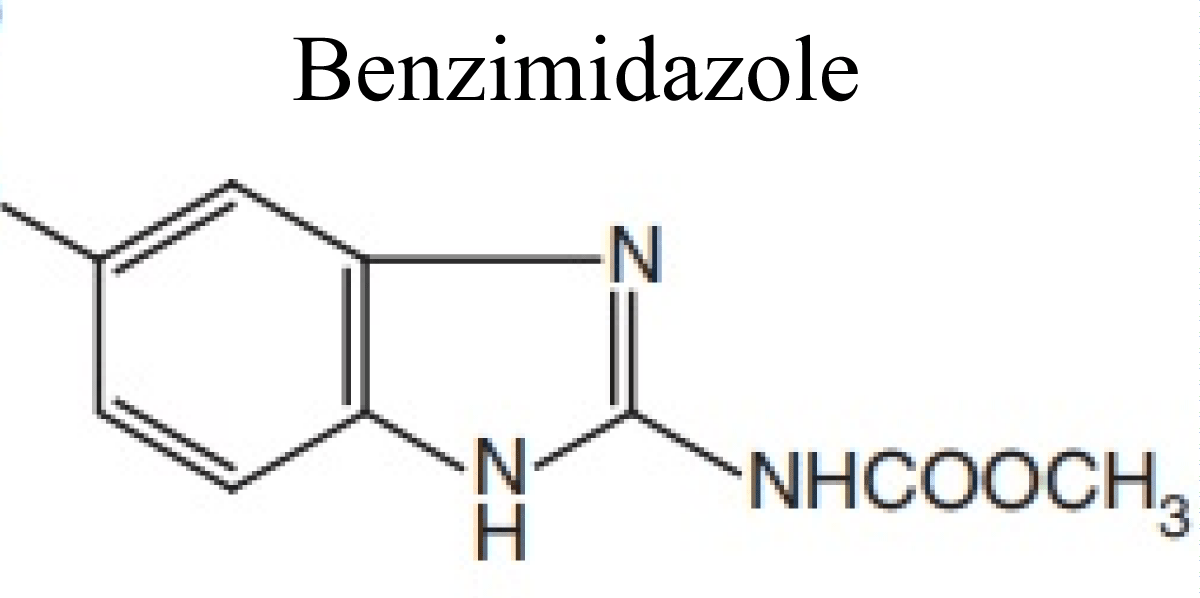
Benzimidazoles (albendazole, mebendazole, flubenda-zole, cyclobendazole thiabendazole, fenbendazole, oxi-bendazole, parbendazole)
Benzimidazole is a heterocyclic aromatic compound. This bicyclic compound perhaps viewed as fused rings of the aromatic compounds benzene and imidazole. Benzimidazole is produced by condensation of o-phenylenediamine with formic acid or the equivalent trimethyl orthoformate (Figure 3).
Figure 3: Chemical structures of Benzimidazoles.
Thiabendazole, mebendazole, and albendazole belong to benzimidazoles group of antihelmenthic medicines. From benzimidazoles group of antihelmenthic, thiabendazole was first discovered in 1961 and already a mentioned number of more benzamidazoles were interpolated as wide spectrum anthelmintics. BZD anthelmintic drugs are extensively metabolized in all mammalian species is considered [21,22].
Mechanism of action: Benzimidazoles blocks the microtubule formation, then the parasite loses its cytoskeleton, motility eventually dies, and also ijuries glucose uptakes and decrease production of ATP and also other than tubulin blockage, benzimidazoles ruptures the energy metabolism of the host [23].
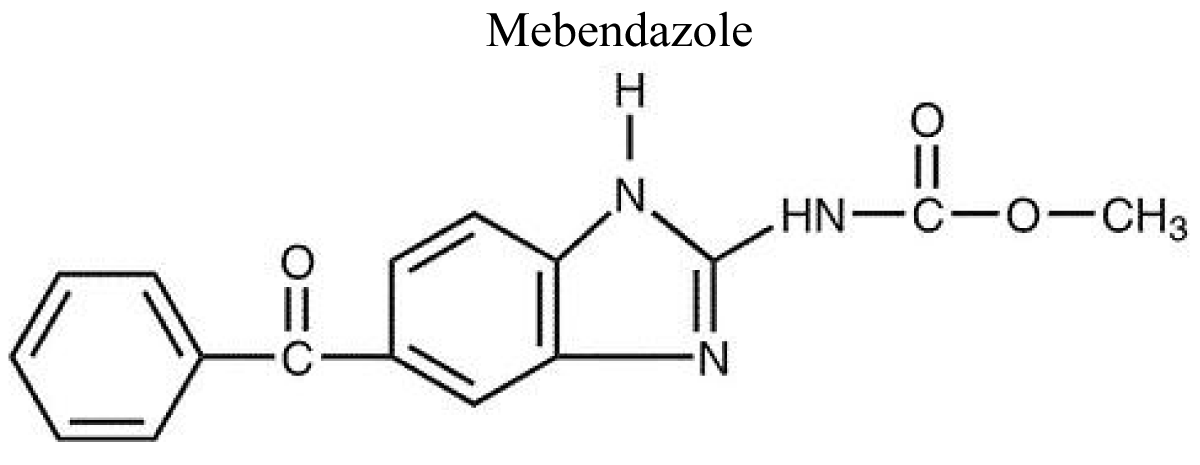
Mebendazole
Mebendazole is a derivative of benzimidazole which is made by reacting 3, 4- diaminobenzophenone with N-methoxycarbonyl-S-methylthiourea (Figure 4).
Figure 4: Chemical structure of Mebendazole.
Mechanism of action: Mebendazole acts selectively and irreversibly to inhibits the uptake of glucose and other nutrients, then leading to autolysis and finally cause parasitic worms die [24].
Use: Mebendazole used for treatment of intestinal worm infections caused by pinworm, hookworm and pinworm, Echinococcus granulosus; nematode infections; capillariasis [25].
Adverse drug reactions: Mebendazole perhaps cause myelosuppression, gastrointestinal disturbances, headache, dizziness, with high doses cause allergic reactions, raised liver enzymes, alopecia, numbness, transient diarrhea [26].
Contraindications: Mebendazole contraindicated in regnant women, for patients who have benzimidazoles hypersensitivity history; for neonates, infants and children less two years [27].
Drug interactions: Coadministration of mebendazole with CYP450 inhibitors medications such as cimetidine, ketoconazole and etc may be increases plasma levels of mebendazole, by extending half-life and decreasing plasma clearance. Concurrent administration of mebendazole with CYP450 inducers medications such as warfarin, phenytoin, carbamazepine etc, decreases the plasma levels of mebendazole by enhancing half-life and increasing plasma clearance [28].
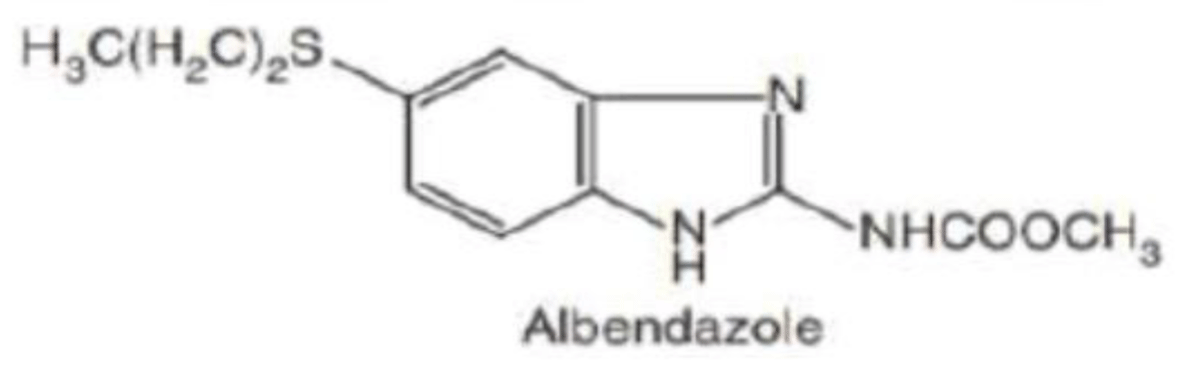
Albendazole
Albendazole is a broad spectrum anthelmintic.
Albendazole, methyl-[5-(propylthio)-1H-benzoimadol-2-yl] carbamate, is made by heterocyclization of a derivative of phenylenediamine to a derivative of benzimidazole [29] (Figure 5).
Figure 5: Chemical structure of Albendazole.
Mechanism of action: Albendazole acts by inhibiting the glucose uptake of larvae. The adult worm stored exhausts of glycogen thus, decreases the formation of ATP, as a sequence the parasite is immobilized and dies. Albendazole inhibits uptake of glucose and other nutrients, leading to autolysis and death of the parasitic worm [30].
Use: Albendazole used for treatment of o variety of parasitic worm infestations such as giardiasis, Trichuriasis, neurocysticercosis, filariasis, pinworm disease and hydatid disease; ascariasis lumbricoides; neurocysticercosis; nematode infections [31].
Adverse drug reactions: Albendazole causes seizures, headache, marrow suppression; yellowing eyes or skin; pregnancy, drug-induced hepatitis, increases in liver enzymes; allergic shock if cyst leakage; convulsions and meningism in cerebral disease; reversible alopecia; rash; and “other” which included spontaneous abortion, change in amount of urine; aplastic anemia; urinary tract infection, motor vehicle accident, dizziness, very stiff neck; fever, vomiting, and intracranial hypertension [32].
Contraindications: Albendazole is not given in pregnant women; for patients who have benzimidazoles hypersensitivity history [33].
Drug interactions: If albendazole coadministered with praziquantel, albendazole activity is more effective due to different mechanism of actions, and praziquantel increases the serum concentration of the active metabolite of albendazole [34].
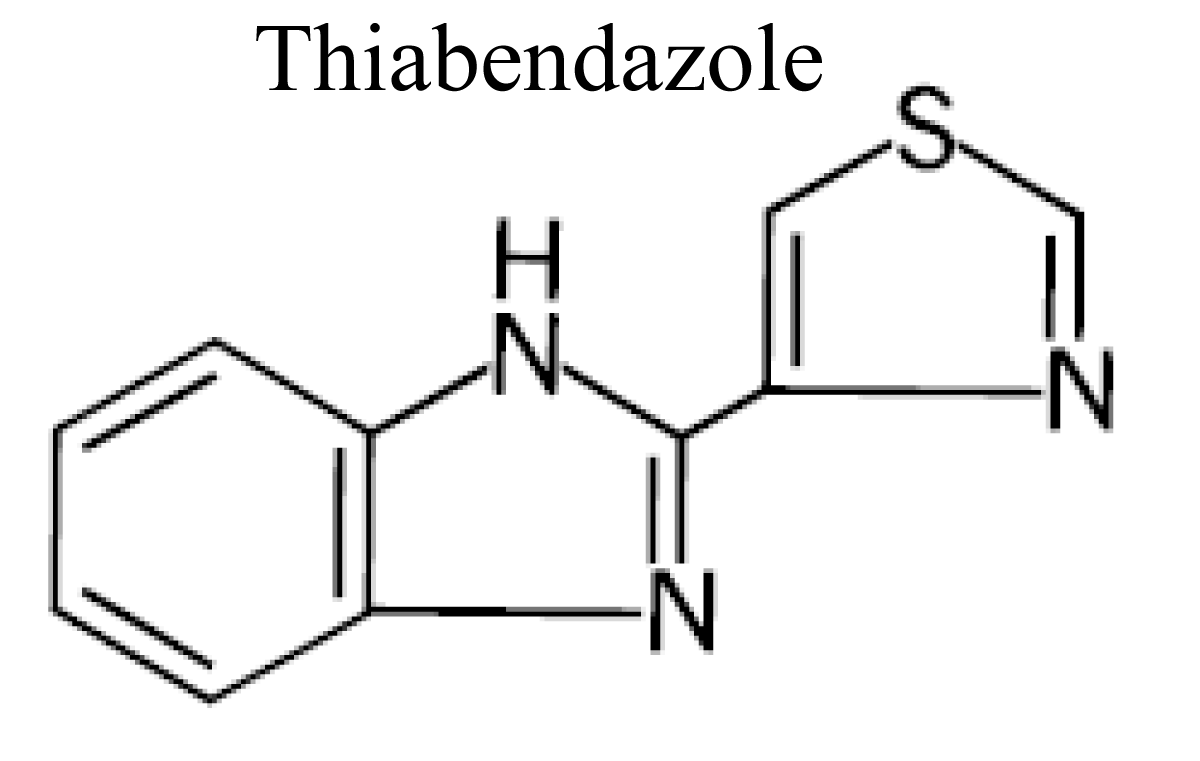
Thiabendazole
Thiabendazole is a broad spectrum anthelmintic.
Thiabendazole is a member of well-known and widely used chemical class of compounds known as the benzimidazoles. It is related in chemical structure and pharmacological properties to other compounds such as fenbendazole, oxibendazole, oxfendazone, febantel or triclabendazole [35] (Figure 6).
Figure 6: Chemical structure of Thiabendazole.
Mechanism of action: Thiabendazole prevents the helminth specific mitochondrial enzyme fumarate reductase by suppressing the citric acid cycle, mitochondrial respiration and subsequent production of ATP, eventually leading to helminths death [36].
Use: Thiabendazole is active against strongyloidiasis, cutaneous larva migrans, and trichinosis.
Currently thiabendazole used rarely because of its higher frequency of side effects when compared with other equally active anthelminthic agents from benzimidazole classes. Thiabendazole is used for the treatment of infections caused by worms like threadworm [37].
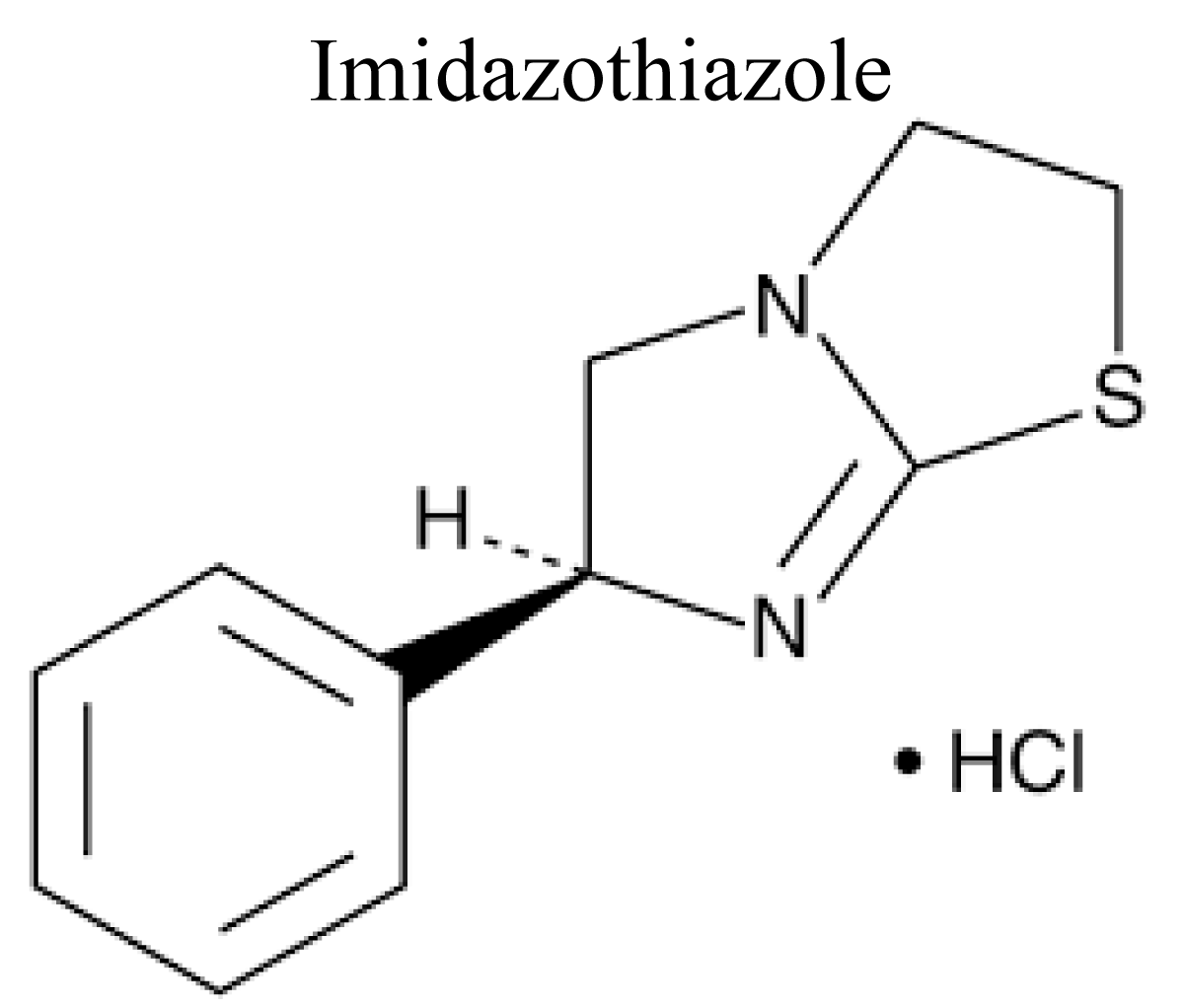
Imidazothiazole (Levamisole, pyrantel and morantel)
Imidazothiazole consisting of an imidazole ring fused with a thiazole ring has been observed to have excellent immunostimulating and anti-inflammatory activity [38] (Figure 7).
Figure 7: Chemical structure of Imidazothiazole.
Levamisole
Levamisole is pure L-isomer of tetramisole and levamisole has been present on the market as long as the benzimidazole drugs, and has also been widely used, it has been displayed that H. contortus has not advanced resistance against levamisole to the identical extent as it has to the benzimidazoles [39].Mechanism of action: Levamisole additional act particularly as agonists at synaptic and extra synaptic nicotinic acetylecholine receptors on nematode muscle cells and generate contraction and spastic paralysis by actin as nicotinic receptor agonists and reduce spastic muscle paralysis of worms due to extended initiation of the excitatory nicotinic acetylcholine (nACh) receptors on body wall muscle [40].
Use: Levamisole is used for treatment of Ascariasis, hookworm, roundworm and mixed ascariasis with hookworm infections; malignant disease; oral ancylostomiasis [41].
Adverse drug reactions: Levamisole cause abdominal discomfort, nausea, vomiting, influenza like syndrome, taste disturbances, thrombocytopenia, rash, muscle pain; dizziness, and headache; agranulocytosis, leukopenia [42].
Contraindications: Levamisole is not given for nursing mother and pregnant women; individuals with severe kidney impairment; previously blood disorders; rheumatoid arthritis [43].
Drug interactions: If levamisole coincidentally given with antiepileptic medications such as phenytoin; it increases the toxicity of antiepileptic medications such as phenytoin. If levamisole administrated with ivermectin, levamisole accelerates the bioavailability of ivermectin [44].
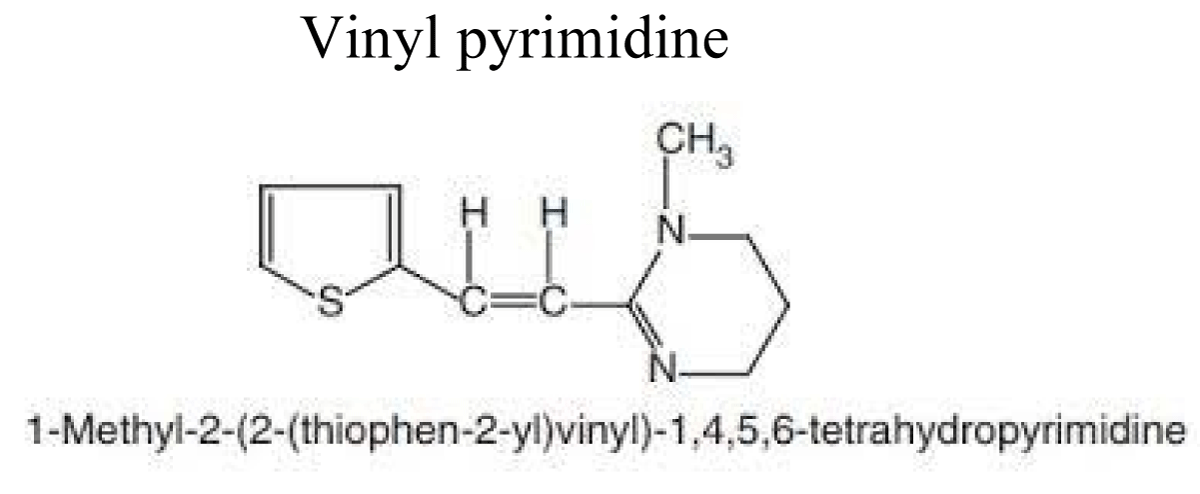
Paraherquamide: Vinyl-pyrimidine (pyrantel, oxantel and its analogues)
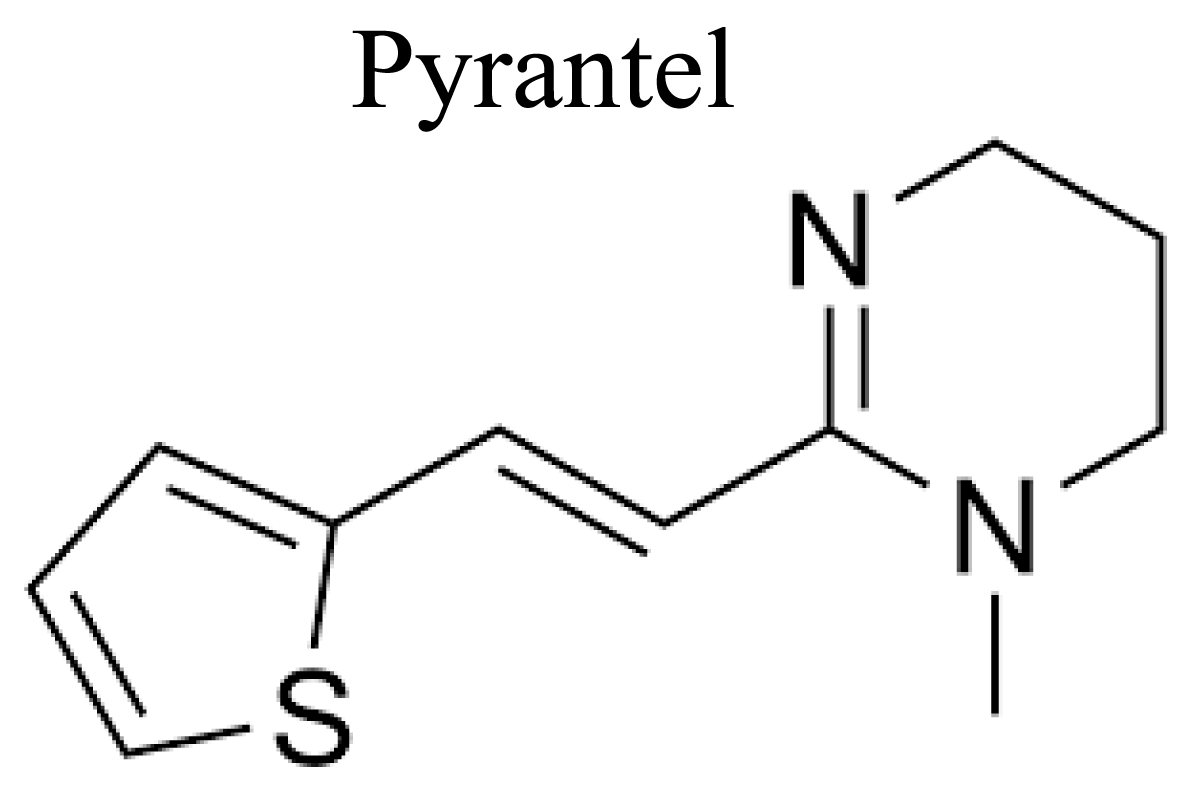
PYRANTELPALMOATE- Pyrantel 1, 4, 5, 6-tetrahydro-1-methyl-2-[trans-2-(2-thienyl) vinyl]-pyrimidine is a derivative of tetrahydropyrimidine which is made from 3-2-thienyl) acrylonitrile from knoevangel of furfural with cyanoacetic acid [45] (Figures 8 & 9).
Figure 8: Chemical structure of Vinyl pyrimidine.
Figure 9: Chemical structure of Pyrantel.
Mechanism of action: Pyrantel is a depolarizing neuromuscular blocking agents and initiates the marked persistent initiation of the nicotinic receptors, which sequence in spastic paralysis of the worm or it generate depolarization, increased spike activity and contraction when functional to ascaris muscle and causes depolarizing type of paralysis (spastic paralysis) of the helminthes [46].
Uses: Pyrantel is used for treatment of ascariasis and enterobiasis, ascariasis, hookworm infections, round worm; pin worm infections; enterobiasis, and trichostrongyliasis; tissue nematode infections [47].
Adverse drug reactions: Pyrantel cause abdominal cramps, headache, insomnia; rash; dizziness, loss of appetite [48].
Ivermectin (macrocylic lactones and milbemycins)
Macrocyclic lactones (avermectin, ivermectin, abamectin) are generated by the genus streptomyces. They can produce a potent and persistent paralysis of nematode pharyngeal and body wall musculature and have broad-spectrum activity against nematodes. They are specifically agonists of glutamate-gated chloride channels, which are continuing only in invertebrates like nematodes and insects. Besides, avermectins also act as antagonists of GABA and nicotinic receptors concreted on somatic muscle cells of parasitic nematodes [49].
Ivermectin
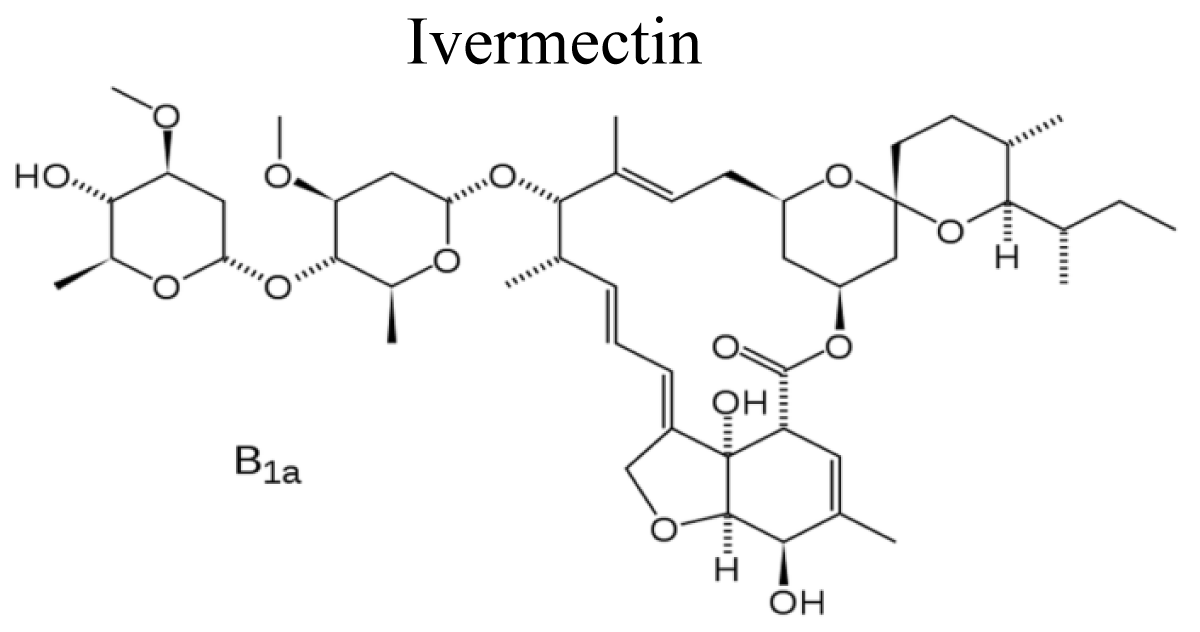
Ivermectin (22.23-dihydroavermectin Bl), a derivative of avermectin A, is a member of the family of substances generated by streptomyces avermitilis. Ivermectin produces a potent and persistent paralysis of nematode pharyngeal and body wall musculature. It has been displayed to interact with a relegate of ligand gated ion channels involving α7 nACh receptors, acetylcholine-gated chloride channels, GABA-gated chloride channels, histamine-gated chloride channels, glycine receptors and P2X4 receptors [50] (Figure 10).
Figure 10: Chemical structure of Ivermectin.
Mechanism of action: Ivermectin selectively binds with great affinity to glutamate-gated chloride ion channels, to increase in the permeability of cell membranes to chloride ion with hyperpolarization of the nerve or muscle cell and ultimately, death of the parasite [51].
Use: Ivermectin indicated for suppressive treatment of onchocerciasis; filariasis; strongyloidiasis; ascariasis lumbricoides; scabies [52].
Adverse drug reactions: Ivermectin cause mild ocular irritation; somnolence; raised liver enzymes; rarely postural hypotension; mild Mazzotti reaction within 3 days of treatment, resulting from death of microfilariae, fever, headache, sore throat, cough, pruritus, rash, conjunctivitis, arthralgia, myalgia, lymphadenopathy, lymphadenitis, oedema, weakness, tachycardia, nausea and vomiting, diarrhoea [53].
Contraindications: Ivermectin not indicated for pregnant women and nursing mother; children less than fifty kilogram body weight [54].
Drug interactions: If ivermectin given with alcohol and levamisole, its bioavailability perhaps increased [55].
Emodepside (cyclodepsipeptides, PF1022A)
The molecule has pore-forming properties in planar lipids; however they does not seem to be significant in giving its anthelmintic potency as an optical isomer of emodepside, with identical pore forming properties, does not have anthelmintic action. Thus it would seem that it perhaps function via stereospecific binding to a receptor [56].
Nitazoxanide
Nitazoxanide is a pyruvate ferredoxin oxidoreductase inhibitor that acts against a wide spectrum of protozoa and helminths that happen in the intestinal tract [57].
Mechanism of action: Nitazoxanide interferes with the pyruvate ferredoxin oxidoreductase enzyme dependent electron transfer reaction which is crucial for anaerobic metabolism [58].
Use: Nitazoxanide is recently used for the treatment of protozoal infections; for C. difficile associated diarrhea [59].
Adverse drug reactions: Nitazoxanide cause diaphoresis; urine discoloration; allergic reactions; anemia; abdominal discomfort; hypertension; tachycardia; headache, dizziness [60].
Drug interactions: Nitazoxanide perhaps interact with highly protein plasma binding medications such as warfarin, phenytoin, methimazole etc because they compete for the same site of binding.
Praziquantel
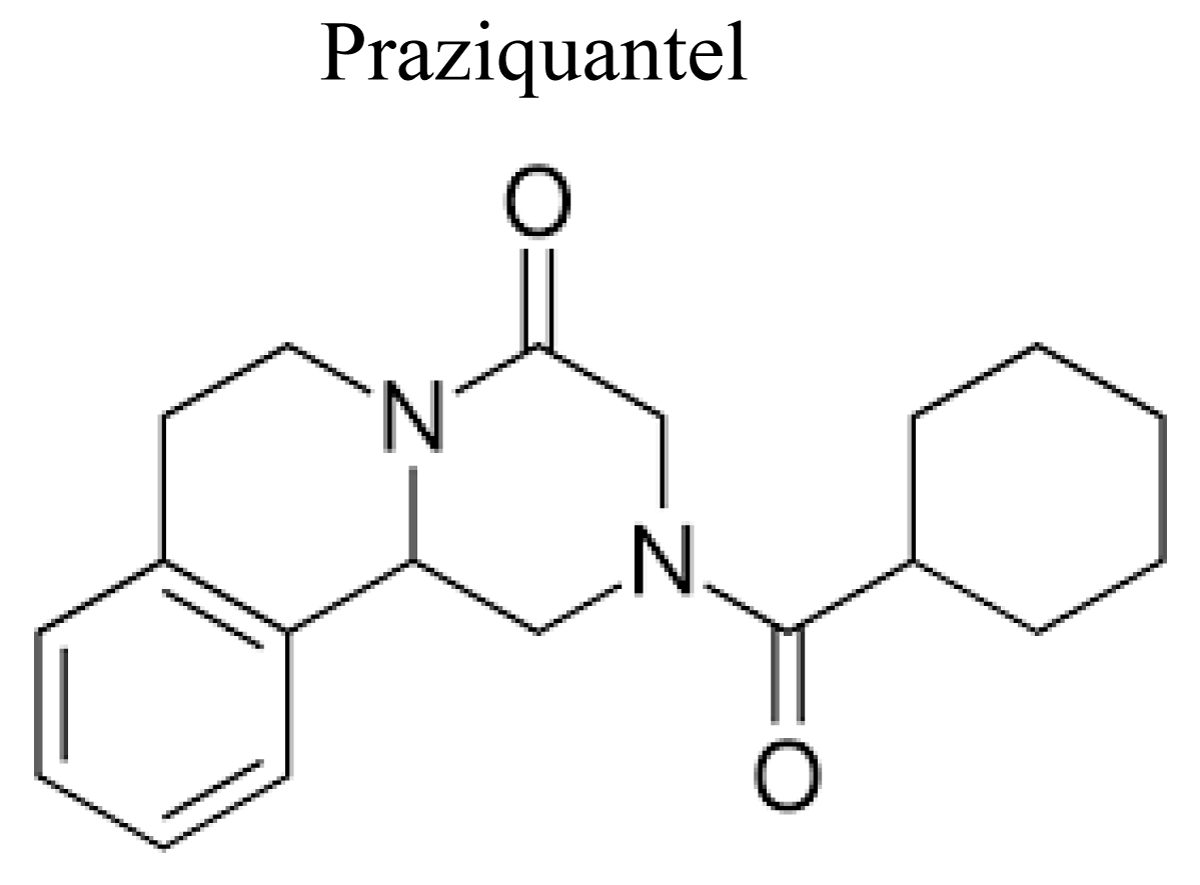
The anthelmintic activity of pyrazinoisoquinoline derivatives was discovered jointly by E. Merck and Bayer AG in 1972. Praziquantel has a selective consequence on the enveloping cover of trematodes and increases permeability of calcium ion influx leading to uncontrolled muscle contraction and paralysis. Praziquantel has a selective toxic effect on schistosome parasites, where its mode of action has been considered more extensively than in cestodes [61] (Figure 11).
Figure 11: Chemical structure of Praziquantel.
Mechanism of action: Praziquantel act by paralyzing worms’ muscular and immobilize their suckers, then cause worms to dislodge from mesenteric veins to the liver, then killed by host tissue reactions or functions by causing strong contractions within the parasite that causes paralysis and eventual dislodgment [62].
Use: Praziquantel indicated for treatment of Taenia saginata, T. solium, Hymenolepis nana and Diphyllobothrium latum infections; trematode infections; schistosomiasis; cysticercosis [63].
Adverse drug reactions: Praziquantel cause abdominal discomfort, nausea, vomiting, diarrhoea, malaise; headache, dizziness, drowsiness; rarely hypersensitivity reactions including fever, urticaria, pruritus, eosinophilia (may be due to dead and dying parasites); in neurocysticercosis, headache, hyperthermia, seizures, intracranial hypertension (inflammatory response to dead and dying parasites in CNS) [64].
Contraindications: Praziquantel is not given for individuals who have ocular cysticercosis; hypersensitive patients to the medications and its derivatives [65].
Drug interactions: If praziquantel administered with antibiotic such as rifampicin concurrently; rifampicin decreases plasma concentrations of praziquantel. If
Praziquantel is given together with chloroquine, carbama-zepine and phenytoin; they decrease the bioavailability of praziquantel. If praziquantel and cimetidine administered concomitantly; cimetidine increases the bioavailability of praziquantel. If praziquantel administrated with dexam-ethasone, dexamethasone decreases the plasma levels concentrations of praziquantel [66].
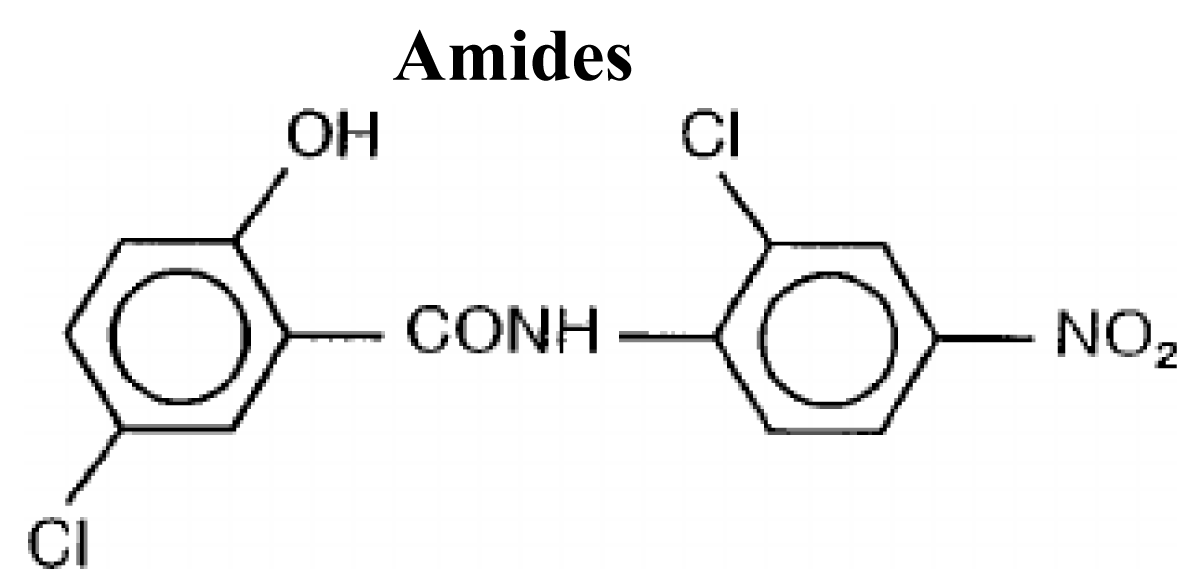
Amides (Niclosamide)
Amides have a general structure in which a nitrogen atom is bounded to a carbonyl carbon atom (Figure 12).
Figure 12: Chemical structures of Amides.
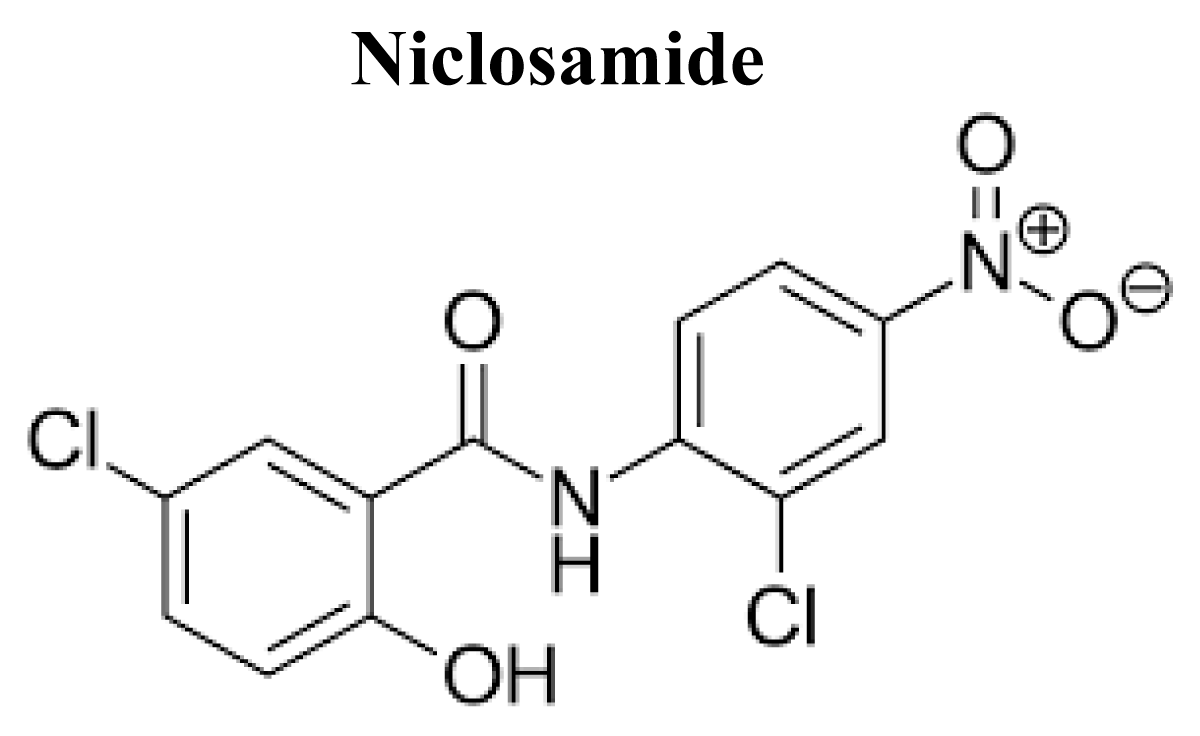
Niclosamide
Niclosamide is a secondary carboxamide resulting from the formal condensation of the carboxy group of 5-chlorosalicylic acid with the amino group of 2-chloro-4-nitroaniline (Figure 13).
Figure 13: Chemical structure of Niclosamide.
Mechanism of action: Niclosamide acts by inhibiting oxidative phosphorylation in mitochondria and interfering with anerobic production of ATP in tapeworm is resulting in energy depletion [67].
Use: Niclosamide is an anthelminthic which is active against most tapeworms, and greatly effective against cestodes infecting man and also used to for treatment of fish tapeworm, dwarf tapeworm and beef tapeworm infection; used for treatment of Taenia saginata, T. solium, Hymenolepis nana, and diphyllobothrium latum infections [68].
Adverse drug reactions: Niclosamide cause nausea, retching, abdominal pain; lightheadedness; pruritus [69].
Helminth infections are one of the most frequently occuring diseases in advancing and advanced countries. Albendazole and mebendazole are selected for mass medicine administration programs and most actively used for treatment of ascariasis and hookworm infections. Anthelmintic drugs are divided into some groups depending on the basis of their identical chemical structure and mode of action. Levamisole is act selectively as agonists at synaptic and extra synaptic nicotinic acetylecholine receptors on nematode muscle cells and generate con-traction and spastic paralysis. Ivermectin indicated for suppressive treatment of onchocerciasis; filariasis; strongyloidiasis; ascariasis lumbricoides; scabies. If albendazole coadministered with praziquantel, albendazole activity is more effective due to different mechanism of actions, and praziquantel increases the serum concentration of the active metabolite of albendazole.
The author would be grateful to anonymous reviewers for the comments that increase the quality of this manuscript.
Data Sources: Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, and Cochrane database. Search terms included: antihelmenthic agents both vermicide and vermifuge.
- Bogitsh BJ, Carter CE, Oeltmann TN. Human parasitology. Academic Press; 2018 May 28.
- Loos-Frank B, Grencis RK. Parasitic worms. The Biology of Parasites. 2016 Dec 16:182.
- Mirisho RO. Intestinal Helminths Infestation in Children Attending Princess Marie Louise Children’s Hospital (Doctoral dissertation, University of Ghana).
- Prakash S, Ranjan R, Pandey RK. Biodiversity and Development.
- Sarwa KK, Vishwakarma PK, Suryawanshi VK, Sahu T, Kumar L, Shree J. An Ayurvedic Formulation Sankat Mochan: A Potent Anthelmintic Medicine. Drug Development & Therapeutics. 2017 Jan 1;8(1).
- Sarwa KK, Vishwakarma PK, Suryawanshi VK, Sahu T, Kumar L, Shree J. An Ayurvedic Formulation Sankat Mochan: A Potent Anthelmintic Medicine. Drug Development & Therapeutics. 2017 Jan 1;8(1).
- Srinivasamurthy SK, Bairy LK. Chemotherapy of Helminthiasis. InIntroduction to Basics of Pharmacology and Toxicology 2021 (pp. 1027-1046).
- McKay DM, Webb RA. Cestode infection: immunological considerations from host and tapeworm perspectives. Parasitic flatworms: molecular biology, biochemistry, immunology and physiology. 2006:193-209.
- Chai JY, Jung BK. Epidemiology of trematode infections: An update. InDigenetic Trematodes 2019;359-409.
- Merachew W, Alemneh T. Review on Triclabendazole Resistance in Fasciola. J Veter Sci Med. 2020;8(1):8.
- Merachew W, Alemneh T, Debela M. Evaluation of the Efficacy of Triclabendazole in Naturally Infected Sheep with Fasciola species at Bonga Sheep Breeding and Improvement Center, South West Ethiopia. International Journal of Veterinary Science and Research. 2020 Dec 17;6(2):178-83.
- International Helminth Genomes Consortium. Comparative genomics of the major parasitic worms. Nat Genet. 2019 Jan;51(1):163-174. doi: 10.1038/s41588-018-0262-1. Epub 2018 Nov 5. PMID: 30397333; PMCID: PMC6349046.
- Subbarayan S, Vadivel B, Krishnamoorthy R, Alshatwi AA. Anthelmintic potentials of Andrographis paniculata: A preliminary study. Scientific Journal of Microbiology. 2017 Mar 24;6(3):149-55.
- Subbarayan S, Vadivel B, Krishnamoorthy R, Alshatwi AA. Anthelmintic potentials of Andrographis paniculata: A preliminary study. Scientific Journal of Microbiology. 2017 Mar 24;6(3):149-55.
- Vinod R, Shailendra W. PHARMACOLOGICAL SCREENING OF ALSOTONIA SCHOLARIS. Asian Journal of Pharmaceutical Research and Development. 2016 Jan 1:1-05.
- Partridge FA, Forman R, Willis NJ, Bataille CJ, Murphy EA, Brown AE, Heyer-Chauhan N, Marinič B, Sowood DJ, Wynne GM, Else KJ. 2, 4-Diaminothieno [3, 2-d] pyrimidines, a new class of anthelmintic with activity against adult and egg stages of whipworm. PLoS neglected tropical diseases. 2018 Jul 11;12(7):e0006487.
- Zhang H, Liu C, Zheng Q. Development and application of anthelminthic drugs in China. Acta Trop. 2019 Dec;200:105181. doi: 10.1016/j.actatropica.2019.105181. Epub 2019 Sep 19. PMID: 31542370.
- Anderson R, Farrell S, Turner H, Walson J, Donnelly CA, Truscott J. Assessing the interruption of the transmission of human helminths with mass drug administration alone: optimizing the design of cluster randomized trials. Parasit Vectors. 2017 Feb 17;10(1):93. doi: 10.1186/s13071-017-1979-x. PMID: 28212667; PMCID: PMC5316156.
- Liu M, Panda SK, Luyten W. Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes. Biomolecules. 2020 Mar 9;10(3):426. doi: 10.3390/biom10030426. PMID: 32182910; PMCID: PMC7175113.
- Trallianus A. 1.1 Historical contemplation on the diversity of parasitic helminths. Analysis of the efficacy of aminophenylamidines and cyclooctadepsipeptides and their mode of action.:20.
- Gaba M, Mohan C. Development of drugs based on imidazole and benzimidazole bioactive heterocycles: recent advances and future directions. Med Chem Res. 2016 Feb 1;25(2):173-210.
- Scott KA, Qureshi MH, Cox PB, Marshall CM, Bellaire BC, Wilcox M, Stuart BAR, Njardarson JT. A Structural Analysis of the FDA Green Book-Approved Veterinary Drugs and Roles in Human Medicine. J Med Chem. 2020 Dec 24;63(24):15449-15482. doi: 10.1021/acs.jmedchem.0c01502. Epub 2020 Oct 30. PMID: 33125236.
- Kaji MD. Investigating the role of species differences and subunit isoforms in sensitivity towards drugs that target nematode ligand-gated ion channels (Doctoral dissertation, McGill University (Canada)).
- Herath HM. Discovery of natural product scaffolds with anthelmintic activity against Haemonchus contortus (Doctoral dissertation).
- Petersen J. Investigation into the Anticancer Mechanism of Action of Novel 4-Substituted Phenylthiazoles and Antihelminthic Benzimidazoles (Doctoral dissertation, University of Otago).
- Argüello-García R, Leitsch D, Skinner-Adams T, Ortega-Pierres MG. Drug resistance in Giardia: Mechanisms and alternative treatments for Giardiasis. Adv Parasitol. 2020;107:201-282. doi: 10.1016/bs.apar.2019.11.003. Epub 2020 Jan 17. PMID: 32122530.
- Hussein SM. Detection of Parasitic Infections and Their Associated Risk Factors in Drinking Water at Basic Schools in Khartoum State-Sudan (Doctoral dissertation, Sudan University of Science & Technology).
- Chai JY, Jung BK, Hong SJ. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: an Update. Korean J Parasitol. 2021 Jun;59(3):189-225. doi: 10.3347/kjp.2021.59.3.189. Epub 2021 Jun 21. PMID: 34218593; PMCID: PMC8255490.
- Son DS, Lee ES, Adunyah SE. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020 Aug 4;20(4):e29. doi: 10.4110/in.2020.20.e29. PMID: 32895616; PMCID: PMC7458798.
- Patil VM, Bhelekar A, Menon N, Bhattacharjee A, Simha V, Abhinav R, Abhyankar A, Sridhar E, Mahajan A, Puranik AD, Purandare N, Janu A, Ahuja A, Krishnatry R, Gupta T, Jalali R. Reverse swing-M, phase 1 study of repurposing mebendazole in recurrent high-grade glioma. Cancer Med. 2020 Jul;9(13):4676-4685. doi: 10.1002/cam4.3094. Epub 2020 May 13. PMID: 32400117; PMCID: PMC7333848.
- Rao NR, Tyagi Y, Sidola S. A Comparative Study Of Different Marketed Formulations Of Albendazole. Indian J Res Pharm Biotechnol. 2020 Mar 28;8(3).
- Melter O, Castelhano R, editors. The MicroBook: Clinical Microbiology for Medical Students. Charles University in Prague, Karolinum Press; 2019 May 1.
- Srinivasamurthy SK, Bairy LK. Chemotherapy of Helminthiasis. InIntroduction to Basics of Pharmacology and Toxicology 2021;1027-1046.
- Légaré D, Ouellette M. Drug resistance assays for parasitic diseases. InAntimicrobial drug resistance 2017;1409-1463.
- Liu M, Panda SK, Luyten W. Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes. Biomolecules. 2020 Mar 9;10(3):426. doi: 10.3390/biom10030426. PMID: 32182910; PMCID: PMC7175113.
- McHugh MA. Ligand-gated ion channels: Putative target sites for anthelmintic therapy in muscle and intestine cells of parasitic nematodes.
- Holden-Dye L, Walker RJ. Anthelmintic drugs and nematicides: studies in Caenorhabditis elegans. WormBook. 2014 Dec 16:1-29. doi: 10.1895/wormbook.1.143.2. PMID: 25517625; PMCID: PMC5402214.
- Deokate U, Lahane Sb, Sujeetkumar A. Review on Anthelmintic Drugs. Int Jo Pharmaceut Res. 2014 Jul;6(3):1.
- Cullin CO, Sellers MS, Rogers ER, Scott KE, Lee DN, Ophir AG, Jackson TA. Intestinal Parasites and Anthelmintic Treatments in a Laboratory Colony of Wild-caught African Pouched Rats (Cricetomys ansorgei). Comp Med. 2017 Oct 1;67(5):420-429. PMID: 28935004; PMCID: PMC5621570.
- Martin RJ, Geary TG. Pharmacology of pyrantel. InPyrantel Parasiticide Therapy in Humans and Domestic Animals 2016 Jan 1;21-45.
- Taman A, Azab M. Present-day anthelmintics and perspectives on future new targets. Parasitol Res. 2014 Jul;113(7):2425-33. doi: 10.1007/s00436-014-3969-7. Epub 2014 Jun 4. PMID: 24894082.
- Al-Hindi AI. Some health and parasitological problems in Gaza strip. The University of Liverpool (United Kingdom); 2010.
- Mackenzie CD. The Safety of Pyrantel, Oxantel, and Morantel. InPyrantel Parasiticide Therapy in Humans and Domestic Animals 2016 Jan 1;47-66.
- Gutierrez KJ, Nutz PA, Albanese JA. Mosby's Nursing Drug Cards E-Book. Elsevier Health Sciences; 2018 Mar 1.
- Liu M, Panda SK, Luyten W. Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes. Biomolecules. 2020 Mar 9;10(3):426. doi: 10.3390/biom10030426. PMID: 32182910; PMCID: PMC7175113.
- Gagman HA. In Vitro Study Of The Efficacy Of Six Ethnoveterinary Plants Species Against Parasitic Nematodes And Caenorhabditis elegans In Nigeria (Doctoral dissertation, Universiti Sains Malaysia).
- McHugh MA. Ligand-gated ion channels: Putative target sites for anthelmintic therapy in muscle and intestine cells of parasitic nematodes.
- Kaji MD. Investigating the role of species differences and subunit isoforms in sensitivity towards drugs that target nematode ligand-gated ion channels (Doctoral dissertation, McGill University (Canada)).
- Deokate U, Lahane SB, Sujeetkumar A. Review on Anthelmintic Drugs. Int J Pharmaceut Res. 2014 Jul;6(3):1.
- Yadav V, Singh SP, Yadav S, Diwakar RP, Gautam AK. Methicillin resistant staphylococcus aureus in animals: a. antimicrobial resistance and future prospects in human and animal health. 78.
- Shakya A, Bhat HR, Ghosh SK. Update on Nitazoxanide: A Multifunctional Chemotherapeutic Agent. Curr Drug Discov Technol. 2018;15(3):201-213. doi: 10.2174/1570163814666170727130003. PMID: 28748751.
- Watkins RR, Eckmann L. Treatment of giardiasis: current status and future directions. Curr Infect Dis Rep. 2014 Feb;16(2):396. doi: 10.1007/s11908-014-0396-y. PMID: 24493628.
- da Silva VBR, Campos BRKL, de Oliveira JF, Decout JL, do Carmo Alves de Lima M. Medicinal chemistry of antischistosomal drugs: Praziquantel and oxamniquine. Bioorg Med Chem. 2017 Jul 1;25(13):3259-3277. doi: 10.1016/j.bmc.2017.04.031. Epub 2017 Apr 27. PMID: 28495384.
- Deokate U, Lahane SB, Sujeetkumar A. Review on Anthelmintic Drugs. Int J Pharmaceut Res. 2014 Jul;6(3):1.
- Greer DM, Beekman RB, Johnson MH, Huttner AJ. Cerebrovascular disease. InAtlas of Clinical Neurology 2019; 167-285.
- Ogilvy KL, Smrčka M, Suwanwela NC, Can U, Ay H, Greenberg SC, Finklestein GR, Foster GP, Koroshetz WJ, Suwanwela N, Kistler JP. Cerebrovascular disease. Atlas Clin Neurol. 2013 Jun 29:121.
- Tuloup V, France M, Garreau R, Bleyzac N, Bourguignon L, Tod M, Goutelle S. Model-Based Comparative Analysis of Rifampicin and Rifabutin Drug-Drug Interaction Profile. Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0104321. doi: 10.1128/AAC.01043-21. Epub 2021 Aug 17. PMID: 34228545; PMCID: PMC8370242.
- Rezaee H, Pourkarim F, Pourtaghi-Anvarian S, Entezari-Maleki T, Asvadi-Kermani T, Nouri-Vaskeh M. Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview. Pharmacol Res Perspect. 2021 Feb;9(1):e00705. doi: 10.1002/prp2.705. PMID: 33421347; PMCID: PMC7796804.
- Custodio JM. Predicting intestinal transporter effects in food-drug interactions and the role of food on drug absorption. University of California, San Francisco; 2008.
- Chen W, Mook RA Jr, Premont RT, Wang J. Niclosamide: Beyond an antihelminthic drug. Cell Signal. 2018 Jan;41:89-96. doi: 10.1016/j.cellsig.2017.04.001. Epub 2017 Apr 4. PMID: 28389414; PMCID: PMC5628105.
- Wilkie MP, Hubert TD, Boogaard MA, Birceanu O. Control of invasive sea lampreys using the piscicides TFM and niclosamide: Toxicology, successes & future prospects. Aquat Toxicol. 2019 Jun;211:235-252. doi: 10.1016/j.aquatox.2018.12.012. Epub 2018 Dec 21. PMID: 30770146.
- Lawrence MJ, Mitrovic D, Foubister D, Bragg LM, Sutherby J, Docker MF, Servos MR, Wilkie MP, Jeffries KM. Contrasting physiological responses between invasive sea lamprey and non-target bluegill in response to acute lampricide exposure. Aquat Toxicol. 2021 Aug;237:105848. doi: 10.1016/j.aquatox.2021.105848. Epub 2021 May 1. PMID: 34274866.
- Loos JA, Dávila VA, Brehm K, Cumino AC. Metformin Suppresses Development of the ascariasis lumbricoides Larval Stage by Targeting the TOR Pathway. Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01808-19. doi: 10.1128/AAC.01808-19. PMID: 32540980; PMCID: PMC7449199.
- Wen LY, Yan XL, Sun FH, Fang YY, Yang MJ, Lou LJ. A randomized, double-blind, multicenter clinical trial on the efficacy of ivermectin against intestinal nematode infections in China. Acta Trop. 2008 Jun;106(3):190-4. doi: 10.1016/j.actatropica.2008.03.007. Epub 2008 Mar 27. PMID: 18452885.
- Martin RJ, Verma S, Levandoski M, Clark CL, Qian H, Stewart M, Robertson AP. Drug resistance and neurotransmitter receptors of nematodes: recent studies on the mode of action of levamisole. Parasitology. 2005;131 Suppl:S71-84. doi: 10.1017/S0031182005008668. PMID: 16569294.
- Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006 Dec;20(14):2606-8. doi: 10.1096/fj.06-6264fje. Epub 2006 Oct 20. PMID: 17056760.
- Greenberg RM. Ca2+ signalling, voltage-gated Ca2+ channels and praziquantel in flatworm neuromusculature. Parasitology. 2005;131 Suppl:S97-108. doi: 10.1017/S0031182005008346. PMID: 16569296.
- Adam I, Elwasila el T, Homeida M. Is praziquantel therapy safe during pregnancy? Trans R Soc Trop Med Hyg. 2004 Sep;98(9):540-3. doi: 10.1016/j.trstmh.2004.01.001. PMID: 15251403.
- Lahane SS. A review on anthelmintic drugs. Int J Pharmaceut Res. July-September 2014;6(3):1-9.